Abstract
Background
To estimate the infectious period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in older adults with underlying conditions, we assessed duration of coronavirus disease 2019 (COVID-19) symptoms, reverse-transcription polymerase chain reaction (RT-PCR) positivity, and culture positivity among nursing home residents.
Methods
We enrolled residents within 15 days of their first positive SARS-CoV-2 test (diagnosis) at an Arkansas facility from July 7 to 15, 2020 and instead them for 42 days. Every 3 days for 21 days and then weekly, we assessed COVID-19 symptoms, collected specimens (oropharyngeal, anterior nares, and saliva), and reviewed medical charts. Blood for serology was collected on days 0, 6, 12, 21, and 42. Infectivity was defined by positive culture. Duration of culture positivity was compared with duration of COVID-19 symptoms and RT-PCR positivity. Data were summarized using measures of central tendency, frequencies, and proportions.
Results
We enrolled 17 of 39 (44%) eligible residents. Median participant age was 82 years (range, 58–97 years). All had ≥3 underlying conditions. Median duration of RT-PCR positivity was 22 days (interquartile range [IQR], 8–31 days) from diagnosis; median duration of symptoms was 42 days (IQR, 28–49 days). Of 9 (53%) participants with any culture-positive specimens, 1 (11%) severely immunocompromised participant remained culture-positive 19 days from diagnosis; 8 of 9 (89%) were culture-positive ≤8 days from diagnosis. Seroconversion occurred in 12 of 12 (100%) surviving participants with ≥1 blood specimen; all participants were culture-negative before seroconversion.
Conclusions
Duration of infectivity was considerably shorter than duration of symptoms and RT-PCR positivity. Severe immunocompromise may prolong SARS-CoV-2 infectivity. Seroconversion indicated noninfectivity in this cohort.
Keywords: COVID-19, infectivity, nursing homes, RT-PCR, SARS-CoV-2
Among 17 nursing home residents with laboratory-confirmed COVID-19, replication-competent virus was isolated >8 days from diagnosis in only 1 severely immunocompromised resident, despite longer median symptom duration and RT-PCR positivity. Seroconversion, assessed in 12 residents, occurred after culture negativity.
As of August 30, 2020, nursing home residents comprised an estimated 3% of all coronavirus disease 2019 (COVID-19) cases in the United States, but they represented over one quarter of all COVID-19 deaths [1, 2]. The disproportionate burden of deaths among nursing home residents suggests unique virus-host dynamics in this population, likely due to their older age and underlying conditions [3, 4]. Although the infectious period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been described in other populations, it is important to understand the natural history and correlates of SARS-CoV-2 infectivity among nursing home residents, to inform infection prevention and control guidance for this population.
The successful isolation of SARS-CoV-2 in cell culture from a clinical specimen is often used as a proxy for infectiousness because it suggests a person is shedding replication-competent virus, which has the potential for transmission. Although the duration of shedding replication-competent virus in mild-to-moderately ill persons has been shown to be less than 10 days from symptom onset, this period has not been assessed prospectively for nursing home residents [5–7]. In one cross-sectional study of nursing home residents with laboratory-confirmed COVID-19, replication-competent virus was isolated ≤9 days from symptom onset [8]. However, there is evidence that shedding of replication-competent virus can continue for as long as 20 days from symptom onset in hospitalized patients with severe illness or immunocompromise [9], both of which might also occur commonly in nursing home residents. Without more informed estimates of the infectious period in nursing home residents, the safe movement of residents within nursing homes and between acute and long-term care facilities remains a challenge. Therefore, we aimed to describe the duration of SARS-CoV-2 infectivity in specimens from nursing home residents obtained over time. We also examined COVID-19 symptoms, reverse-transcription polymerase chain reaction (RT-PCR) positivity, and serologic status as potential correlates of ongoing infectivity.
METHODS
Participant Identification, Enrollment, and Follow-Up
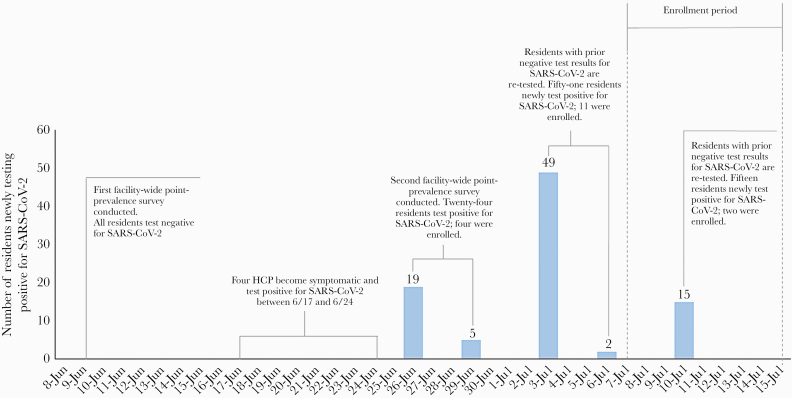
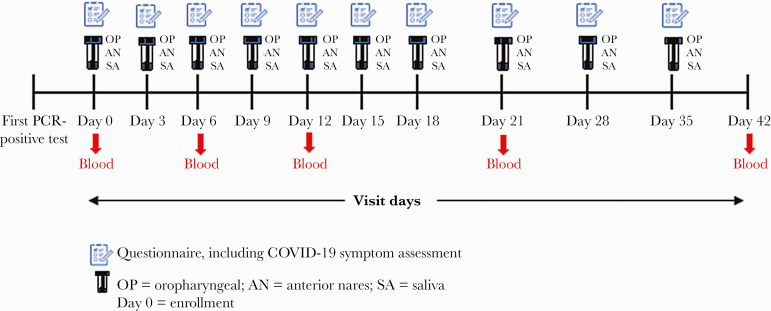
We prospectively followed residents with SARS-CoV-2 infection confirmed by RT-PCR during a nursing home outbreak in rural Arkansas. As part of state-supported, facility-wide testing for early detection of SARS-CoV-2 infection among nursing homes residents and healthcare personnel (HCP), residents with SARS-CoV-2 infection were identified by RT-PCR through serial point-prevalence surveys (PPS) conducted at the facility from June 9, 2020 through July 15, 2020 (Figure 1). To describe the natural history of SARS-CoV-2 infectivity in participants who were both close to and farther out from their first RT-PCR-positive result (or diagnosis), we enrolled residents within 15 days of this result (Figure 1). Each participant was followed for a period of 42 days from enrollment. For the first 21 days, participants were visited every 3 days; for the next 21 days, participants were visited weekly (Figure 2).
Figure 1.
Timeline showing number of nursing home residents (N = 95) newly testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after serial point-prevalence surveys, notable events, and period of enrollment at the nursing home—Arkansas, June 9–July 15, 2020. HCP, healthcare personnel.
Figure 2.
Timeline of visits and activities conducted at each visit, including questionnaire administration and specimen collection. COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Symptom assessment, medical chart review, and specimen collection were repeated at each visit according to the project timeline (Figure 2). Before enrollment, the following symptoms were assessed twice daily by facility HCP: shortness of breath, cough, malaise, muscle pain, dizziness, diarrhea, vomiting, sore throat, and headache. At enrollment and each subsequent visit, participants were interviewed by project staff about COVID-19 symptoms at the time of interview using the Centers for Disease Control and Prevention (CDC) standard list of symptoms, to which chest and abdominal pain were added [10]. Medical charts were reviewed to abstract information on underlying conditions, medications, vital signs (temperature and oxygen saturation), laboratory test results within the previous 30 days, and any hospitalization records from the previous 30 days. At each visit, collection of respiratory specimens (ie, oropharyngeal and anterior nares) and saliva specimens was attempted (Figure 2). Collection of blood for serology was attempted at enrollment and at visit days 6, 12, 21, and 42 (Figure 2). Patients hospitalized during the assessment period were not interviewed and did not have specimens collected during their hospitalization; however, upon return to the nursing home, participants could choose to continue participating in the assessment.
Definitions
Severe acute respiratory syndrome coronavirus 2 infectivity was defined as isolation of replication-competent virus from a specimen in cell culture. Date of diagnosis was the date of participants’ first PCR-positive test. Symptom data collected by the facility and CDC staff were used to determine the earliest date of symptom onset for participants. Although collection of oropharyngeal, anterior nares, and saliva specimens was attempted at each visit, RT-PCR results for each specimen type at each visit were not always available due to challenges with specimen collection, transport, or processing. Thus, we used any positive RT-PCR result among all specimens obtained from a participant on the same day to determine a composite RT-PCR result for each visit. Seroconversion was defined as a signal threshold >1 at the 1:100 dilution. Severe illness, based on adaptation from CDC guidance, was defined as a decrease from baseline of >3% in oxygen saturation (SpO2) regardless of whether the participant was on room air or supplemental oxygen [11].
Specimen Collection and Processing
All specimens from participants’ first RT-PCR-positive test before enrollment were collected during routine PPS conducted at the facility and tested at laboratories external to CDC. Residual specimen from the first RT-PCR-positive test for each participant was requested from these external laboratories and, if available, retested at the CDC to determine whether specimen quality would support performing culture. For specimens taken at enrollment and subsequently, oropharyngeal and anterior nares specimens were collected using synthetic swabs (BD NS Regular Flocked Swab, Franklin Lakes, NJ) and preserved in 3 mL viral transport media. Saliva was collected in OMNIGene·ORAL kits (DNA Genotek, Ottawa, Ontario, Canada). Blood was collected in K2 EDTA tubes (BD Vacutainer Plastic Blood Collection Tubes: Hemogard Closure; Franklin Lakes, NJ). All specimens were kept at a temperature of 2–8°C during transport. Respiratory specimens and saliva were tested using the CDC 2019-Novel Coronavirus RT-PCR Diagnostic Panel [12].
All oropharyngeal or anterior nares specimens with a positive RT-PCR result were stored at −70°C and submitted for viral culture within 4 weeks of RT-PCR testing. Reverse-transcription polymerase chain reaction-positive saliva specimens could not be cultured because the transport media inactivated the virus [13]. Viral culture was conducted by placing 100 µL of specimen in a 96-well plate in serum-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2× penicillin-streptomycin and 2× amphotericin B (Sigma-Aldrich, St. Louis, MO). Vero CCL-81 cells were trypsinized and resuspended in DMEM + 10% fetal bovine serum + 2× penicillin-streptomycin + 2× amphotericin B at 2.5 × 105 cells per mL. A 100-µL cell suspension was added directly to the clinical specimen dilutions and mixed gently by pipetting. The inoculated cultures were grown in humidified 37°C incubator with 5% CO2 and observed for cytopathic effect daily. When cytopathic effect was observed, presence of SARS-CoV-2 was confirmed by RT-PCR.
Serum samples were analyzed using a validated pan immunoglobulin (Ig) enzyme-linked immunosorbent assay against the prefusion-stabilized extracellular domain of the SARS-CoV-2 spike protein to detect anti-SARS-CoV-2 antibodies for IgM, IgA, and IgG [14]. A specimen was considered reactive if the signal-to-threshold ratio at a serum dilution of 1:100 with background correction was greater than 1.0.
Sample Size, Inclusion and Exclusion Criteria, and Analyses
We used a convenience sample of residents with RT-PCR-confirmed SARS-CoV-2 infection. Residents were eligible for inclusion if they were within 15 days of their COVID-19 diagnosis. Residents were excluded if they had died, were hospitalized at the time of enrollment, did not have capacity to make independent decisions, or had their initial RT-PCR-positive test >15 days before enrollment. Data were summarized by measures of central tendency, frequencies, and proportions using SAS software version 9.4 (SAS Institute, Cary, NC).
Patient Consent Statement
Participation in this activity was voluntary, and all participants were informed about procedures, risks, benefits, and provided written consent. This activity was reviewed by the CDC and was determined to be nonhuman subjects research as part of public health response, consistent with applicable federal law and CDC policy (see, eg, 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq).
RESULTS
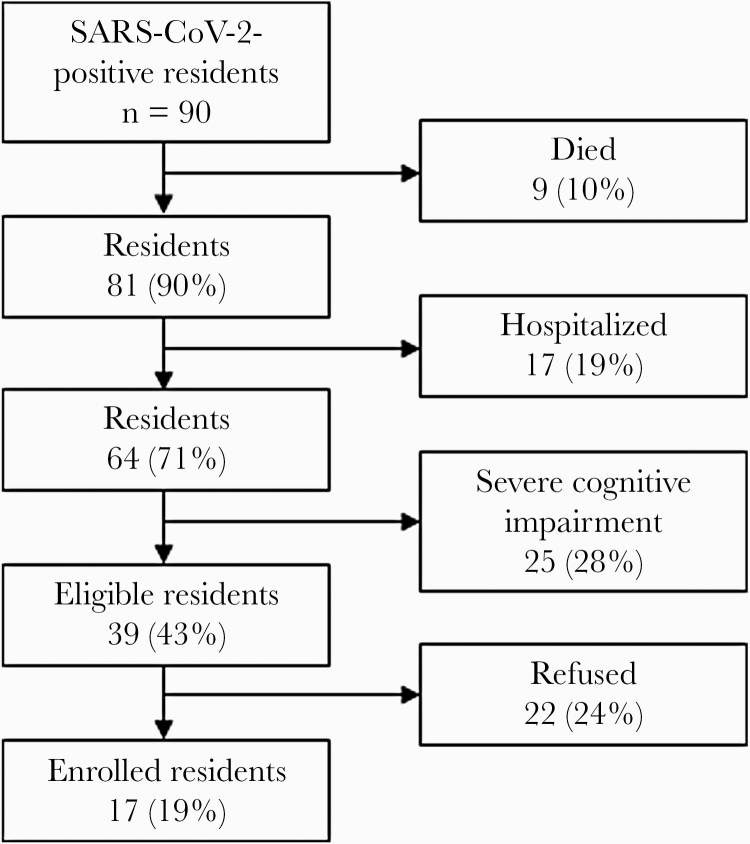
Of 90 residents at the facility with SARS-CoV-2 infection confirmed by RT-PCR, 39 (43%) were eligible for enrollment and 17 of 39 (44%) were enrolled (Figure 3). Median age of participants was 82 years (range, 58–97 years) and a majority (10, 59%) were female (Table 1). All participants had ≥3 underlying conditions, most commonly the following: cardiovascular disease (16, 94%), diabetes (7, 41%), and nonasthmatic chronic lung disease (7, 41%) (Table 1). Of 17 participants, 5 (29%) were classified as having severe COVID-19 illness. Four (24%) participants were hospitalized; 3 continued participating after discharge. There were 3 (18%) deaths; 2 of these were thought by the primary care physician to be COVID-19-related.
Figure 3.
Participant enrollment among all residents (N = 95) at the nursing home—Arkansas, July 7–15, 2020. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Table 1.
Demographic and Clinical Characteristics of Participants (N = 17) with COVID-19 at the Nursing Home—Arkansas, July 2020
| All Participants | ||
|---|---|---|
| Characteristic | n | % |
| Age, median [range] in years | 82 | [58–97] |
| Female | 10 | 59 |
| White race | 17 | 100 |
| Non-Hispanic/Latino | 17 | 100 |
| Former or current tobacco use | 10 | 59 |
| Former or current alcohol use | 8 | 47 |
| Underlying Conditions | ||
| ≥3 underlying conditions | 17 | 100 |
| Cardiovascular disease | 16 | 94 |
| Hypertension | 14 | 82 |
| Coronary artery disease | 6 | 35 |
| Hyperlipidemia | 7 | 41 |
| Heart failure | 7 | 41 |
| Cerebrovascular accident | 3 | 18 |
| Diabetes mellitus | 7 | 41 |
| Nonasthmatic chronic lung disease | 7 | 41 |
| Chronic obstructive pulmonary disease | 6 | 35 |
| Chronic bronchitis | 1 | 6 |
| Neurologic disorder | 4 | 24 |
| Chronic kidney disease | 2 | 12 |
| Cancera | 1 | 6 |
| Liver disease | 0 | 0 |
| Symptoms at Onsetb | ||
| Cough | 8 | 47 |
| Dyspnea | 5 | 29 |
| Fatigue | 5 | 29 |
| Myalgias | 5 | 29 |
| Rhinorrhea | 2 | 12 |
| Sore throat | 2 | 12 |
| Nausea/vomiting | 2 | 12 |
| Diarrheac | 2 | 12 |
| Abdominal pain | 1 | 6 |
| Headache | 1 | 6 |
| Anosmia | 1 | 6 |
| Fever (≥100oF)d | 1 | 6 |
| Subjective fever | 0 | 0 |
| Dysgeusia | 0 | 0 |
| Chills | 0 | 0 |
| Chest pain | 0 | 0 |
| Severe COVID-19 illnesse | 5 | 29 |
| Hospitalized | 4 | 24 |
| Died | 3 | 18 |
Abbreviations: COVID-19, coronavirus disease 2019.
aParticipant had chronic lymphocytic leukemia and was receiving targeted therapy with Ibrutinib, a tyrosine kinase inhibitor, per medical record review.
bSymptoms at onset represent the earliest reported symptoms from 10 days before first reverse-transcription polymerase chain reaction (RT-PCR)-positive test date through study period. Symptoms before enrollment were assessed by healthcare personnel at the facility using a standard symptom list that was different from the symptom assessment used by study staff after enrollment.
cDiarrhea was defined as ≥3 loose stools in 24 hours.
dThe highest recorded temperature at the nursing home was 101.6oF.
eSevere COVID-19 illness was defined as having an oxygen saturation (SpO2) decrease of >3% from baseline from first RT-PCR-positive test date through the study period. For the 5 participants with severe COVID-19 illness, the median SpO2 recorded at the nursing home was 91% (range: 79%–96%).
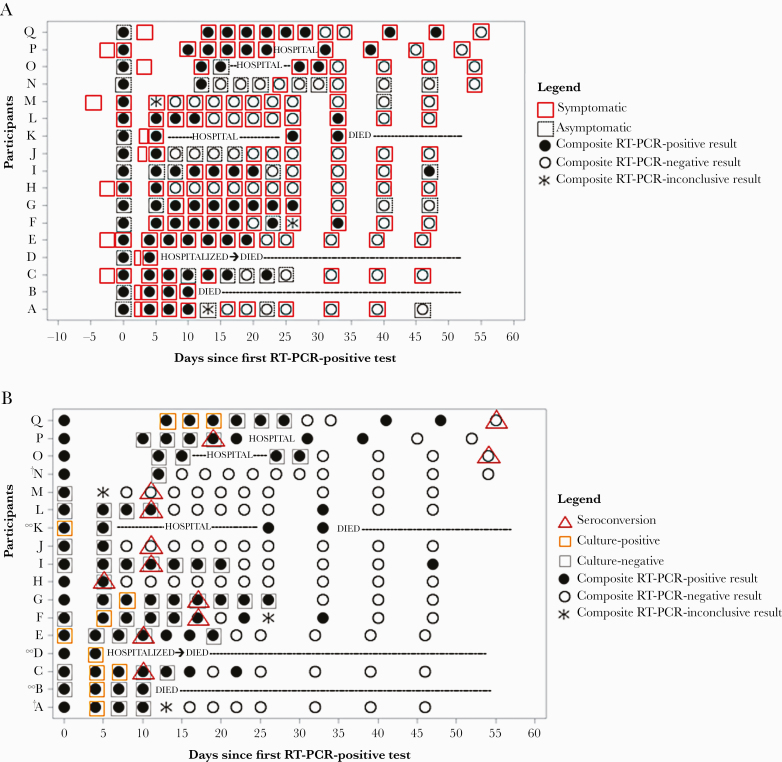
At their first RT-PCR-positive test, 11 (65%) participants had not reported any COVID-19 symptoms, but all eventually became symptomatic (Figure 4A). The most frequently reported symptoms at onset were cough (8, 47%), dyspnea (5, 29%), fatigue (5, 29%), and myalgias (5, 29%) (Table 1). Symptoms were reported for as long as 53 days, with a median duration of 42 days (interquartile range [IQR], 28–49 days). The median duration of respiratory symptoms was 44 days (IQR, 28–50 days), which was longer than the median duration of nonrespiratory symptoms at 36 days (IQR, 24–50 days). The most frequently reported symptoms at the end of the symptomatic (or study) period were fatigue (10, 59%), cough (9, 53%), rhinorrhea (8, 47%), dyspnea (8, 47%), and headache (6, 35%). Symptoms were distributed across the assessment period without any obvious clustering closer to diagnosis and with 8 (47%) participants reporting symptoms intermittently (Figure 4A).
Figure 4.
Period of COVID-19 symptoms (A) and detection of SARS-CoV-2 RNA by RT-PCR, viral culture, and seroconversion (B) among participants (N = 17) at nursing home — Arkansas, July–August 2020. Notes: (1) RT-PCR-positive result at day 0 represents initial diagnosis; (2) Composite RT-PCR results are determined from attempted collection of oropharyngeal, anterior nares, and saliva specimens at each visit. Due to challenges with specimen collection, transport, and processing, RT-PCR results for each specimen type were not always available for each visit. (3) Seroconversion was determined by a signal threshold >1 at the 1:100 dilution. Figure 4B notes: †Participants A and N could not be phlebotomized; ∞Participants B, D, and K all had blood collections at enrollment, but subsequent collections were missed due to refusal, hospitalization, or death.
From the date of first RT-PCR-positive test, the median duration of SARS-CoV-2 ribonucleic acid (RNA) detection by RT-PCR among oropharyngeal, anterior nares, and saliva specimens was 22 days (IQR, 8–31 days) with a range of 1–47 days. Using these same specimens, but measuring from symptom onset, the median duration of SARS-CoV-2 RNA detection by RT-PCR was 20 days (IQR, 7–28 days) with a range of 1–45 days.
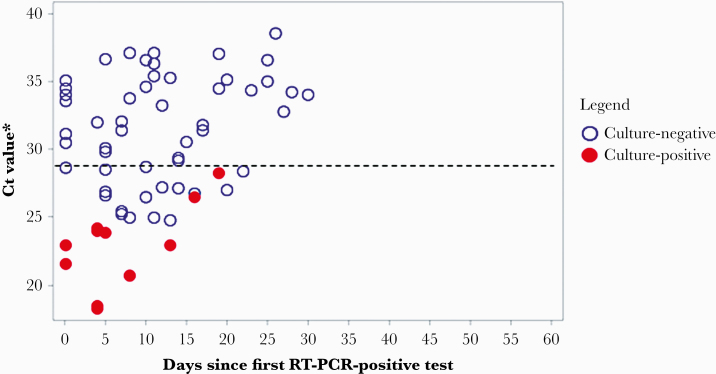
Across all 17 participants, 9 (53%) had replication-competent virus isolated from ≥1 of their specimens. Of these, 1 (11%) severely immunocompromised participant shed replication-competent virus for 19 days from first RT-PCR-positive test (or 17 days from symptom onset), whereas 8 (89%) participants were culture-positive ≤8 days from first RT-PCR-positive test (Figure 4B). Replication-competent virus was not isolated from specimens with a SARS-CoV-2 N1 gene cycle threshold (Ct) value above 29 (Figure 5).
Figure 5.
Viral culture results of reverse-transcription polymerase chain reaction (RT-PCR)-positive oropharyngeal and anterior nares specimens by cycle threshold (Ct) value and days since first RT-PCR-positive result among participants (N = 17) at nursing home—Arkansas, July–August 2020. *The lowest Ct value for N1 is displayed from a composite of oropharyngeal and anterior nares specimens for each participant. --- Indicates a Ct value of 29, above which replication-competent virus could not be cultured.
Blood could only be obtained from 15 of 17 (88%) participants at enrollment and throughout the assessment period. In addition, 3 participants died during follow-up, preventing subsequent blood collections. Of the remaining 12 participants, all seroconverted with seroconversion occurring a median of 11 days after first RT-PCR-positive test (IQR, 5–14 days). Although all participants with culture results available were culture-negative by the time of seroconversion, some participants had a substantial delay between becoming culture negative and seroconversion (Figure 4B).
Participant Q had the longest duration of both RT-PCR positivity (47 days) and culture positivity (19 days) (Figure 4B). Seroconversion was detected 54 days from her first RT-PCR-positive test. Participant Q was the oldest surviving participant in the cohort at 93 years of age. She was severely immunocompromised with chronic lymphocytic leukemia and was receiving targeted therapy with ibrutinib, a tyrosine kinase inhibitor, throughout the assessment period. By contrast, participant M had the shortest duration of RT-PCR positivity, detected only at diagnosis. Replication-competent virus could not be isolated from participant M’s first RT-PCR-positive specimen, but seroconversion was detected 11 days after his first RT-PCR-positive test. Participant M, at 58 years, was the youngest person in the cohort and had only hypertension as a high-risk underlying condition.
DISCUSSION
We prospectively measured the duration of COVID-19 symptoms, RT-PCR positivity, culture positivity, and timing of seroconversion in a nursing home cohort with laboratory-confirmed SARS-CoV-2 infection to assess infectivity among older adults. In this cohort, the duration of infectivity was considerably shorter than the duration of symptoms or RT-PCR positivity. Apart from 1 severely immunocompromised participant who remained culture-positive for 19 days, all other participants in whom culturable virus was isolated were culture-positive ≤8 days from first RT-PCR-positive test, despite having persistent symptoms and RT-PCR-positive results. In addition, seroconversion occurred only after culture negativity, suggesting that becoming potentially noninfective in this cohort is linked to immune response. The period of SARS-CoV-2 infectivity observed for most older adults in this cohort (ie, ≤8 days from first PCR-positive test) is similar to the ≤9-day period of infectivity observed in other populations [5–7], although severe immunocompromise remains an important consideration to determine the duration of transmission-based precautions [15].
The median duration of RT-PCR positivity since symptom onset in this cohort was 20 days. This is slightly longer than the mean duration of 17 days from symptom onset found in a meta-analysis of 43 studies that examined viral RNA detection by RT-PCR in upper respiratory tract specimens [7]. In the meta-analysis, older age correlated with longer durations of viral RNA detection, which might explain the longer duration seen in our older cohort [7]. Despite prolonged RT-PCR positivity in this cohort, the period of infectivity for most participants was shorter and consistent with other reports [7–9]. A cross-sectional study of 48 nursing home residents with confirmed COVID-19 did not isolate culturable virus beyond 9 days from symptom onset, although none of the residents in the study had any severely immunocompromising conditions [8]. By contrast, in a study of 129 hospitalized patients who had severe and critical COVID-19 and were severely immunocompromised, replication-competent virus was isolated up to 20 days, although the probability decreased to <5% after 15 days [9]. Such findings, including evidence of prolonged infectivity in a participant in our cohort with cancer, support the need to continue transmission-based precautions for extended periods in persons who are severely immunocompromised [15].
It is important to note that the isolation of replication-competent virus only indicates the potential for onward transmission. At the time of writing, no late-linked transmissions (after a patient has had symptoms for approximately 1 week) have been documented, despite isolation of culturable virus beyond a week from symptom onset [16]. However, data from a household transmission study in the United States found that household contacts of immunocompromised patients with COVID-19 had increased risk for infection, suggesting that immunocompromised individuals may be more likely to transmit the virus [17]. A plausible explanation for an increased ability to transmit the virus includes prolonged shedding of replication-competent virus.
We were unable to isolate replication-competent virus from specimens with an N1 Ct value above 29. Previous studies that have examined the relationship between Ct values and recovery of culturable virus have not been able to isolate replication-competent virus above Ct values of 24 or 34 [8, 18, 19]. Although our Ct value finding adds to the understanding of Ct values as correlates of the presence of culturable virus, the molecular assays used in these studies were not quantitative, and Ct value does not indicate a direct quantity of virus. The Ct values vary across PCR assays, and, even when the same PCR assay is used, different Ct values can be obtained by different institutions [18–20].
Only approximately one third of our cohort reported symptoms before their first RT-PCR-positive test. After symptom onset, more than half of the participants continued to report respiratory symptoms for >44 days (or nonrespiratory symptoms for >36 days), far longer than the ≤8-day period of infectivity observed in this cohort, suggesting that symptoms do not correlate well with infectivity in older adults. Prolonged symptoms in this older cohort might be expected, given that even young, nonhospitalized adults with no or few underlying conditions have been shown to experience prolonged symptoms after SARS-CoV-2 infection [21].
This assessment has several limitations. First, we used viral culture as a proxy for infectiousness. Although successful virus isolation implies infectiousness, the inability to culture virus cannot be assumed to mean that an individual is not infectious. Several factors may affect detection of replication-competent virus in cell culture, including viral load, limit of detection, and type of cell line [5, 22].
Second, determining symptom onset for COVID-19 in nursing home residents is challenging. Patients often had difficulty distinguishing acute from chronic symptoms, especially for nonspecific symptoms such as fatigue and myalgia. Furthermore, because data collected before enrollment used the facility’s symptom assessment tool, which did not include all symptoms tracked during our assessment, it is possible early symptoms could have been missed, which could have affected length-of-time measurements that were based on symptom onset.
Third, because we did not collect daily blood samples, detection of seroconversion in this cohort is likely delayed. Thus, although seroconversion only occurred after culture negativity in this cohort, data from larger cohorts with daily blood collections that are also tested for neutralization titers are needed to determine the utility of seroconversion as a diagnostic correlate of noninfectivity.
Fourth, the low participation rate could have biased our sample towards residents who are healthier than those who were already hospitalized or had died before enrollment. Finally, our small sample size of 17 residents, entirely comprising non-Hispanic whites from a single facility, limits the generalizability of these results. Despite these limitations, our findings are consistent with other assessments of SARS-CoV-2 infectivity [5–9, 18].
CONCLUSIONS
This assessment is the first to prospectively compare isolation of replication-competent virus in cell culture with detection of SARS-CoV-2 RNA by RT-PCR in a nursing home cohort in the United States. Our findings suggest that older patients are likely to remain symptomatic and carry detectable viral RNA beyond the presence of culturable virus. For these patients, there may be limited value in using symptom resolution and RT-PCR to determine whether transmission-based precautions should be discontinued. In contrast, a time-based approach, based on underlying risk factors as specified in current guidance, would have performed reliably in this cohort. Severe immunocompromise remains an important criterion for prolonged carriage of replication-competent virus and likely necessitates continuation of transmission-based precautions for longer periods of time. Additional studies could assess whether seroconversion can be used as a marker of noninfectivity in this population.
Acknowledgments
We are grateful to all residents and staff at the nursing home for their participation and support of this assessment during an especially challenging time. We also thank the staff at Arkansas State Public Health Laboratory and Centers for Disease Control and Prevention COVID Surge Testing Laboratories for their support.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This assessment has been funded by the US Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Centers for Disease Control and Prevention. CDC COVID Data tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#cases_totalcases. Accessed 13 September 2020.
- 2. Centers for Medicare and Medicaid Services. COVID-19 nursing home data. Available at: https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg/. Accessed 13 September 2020.
- 3. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 6. Kujawski SA, Wong KK, Collins JP, et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med 2020; 26:861–8. [DOI] [PubMed] [Google Scholar]
- 7. Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2 viral load dynamics, duration of viral shedding and infectiousness—a living 2 systematic review and meta-analysis. Lancet Microbe 2021; 2:e13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. Natur Commun 2021; 12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Symptoms. Coronavirus 2019 (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 20 September 2020.
- 11. Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (Interim Guidance). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed 28 September 2020.
- 12. Centers for Disease Control and Prevention. CDC 2019-novel Coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. Available at: https://www.fda.gov/media/134922/download. Accessed 21 October 2020.
- 13. DNAgenotek™. Inactivtion of SARS-CoV-2 in samples collected using Oragene®, ORAcollect®, and OMNIgene® products from DNAgenotek™. Available at: https://www.dnagenotek.com/US/pdf/MK-01430.pdf. Accessed 21 October 2020.
- 14. Freeman B, Lester S, Mills L, et al. Validation of a SARS-CoV-2 spike protein ELISA for use in contact investigations and serosurveillance [preprint]. bioRxiv 2020. [Google Scholar]
- 15. Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (Interim Guidance). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html#definitions. Accessed 21 October 2020.
- 16. Meyerowitz EA, Richterman A, Gandhi RT, Paul S. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med 2020; doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis NM, Chu VT, Ye D, et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1166. [DOI] [Google Scholar]
- 18. Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020; 39:1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nalla AK, Casto AM, Huang MW, et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J Clin Microbiol 2020; 58:e00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tenforde MW, Kim SS, Lindsell CJ, et al. ; IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]