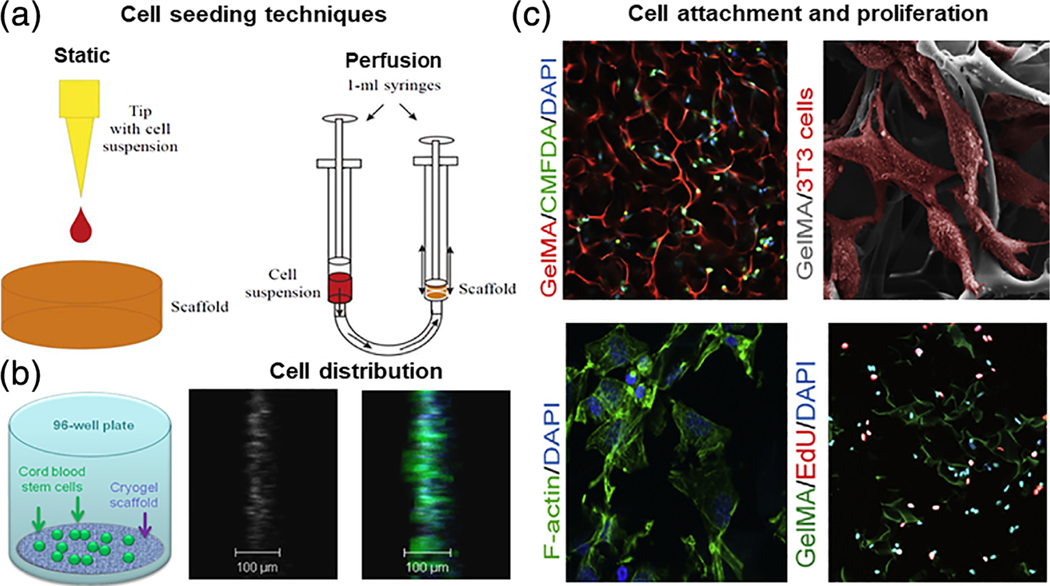
FIGURE 5.
(a) Schematic representation of different techniques for cryogel bioscaffold cell-seeding include static and perfusion (dynamic) methods (reproduced with permission from Petrenko, Y. A., Ivanov, R. V., Lozinsky, V. I., & Petrenko, A. Y. (2011). Comparison of the methods for seeding human bone marrow mesenchymal stem cells to macroporous alginate cryogel carriers. Bulletin of Experimental Biology and Medicine, 150, 543–546); (b) schematic of artificial cryogel bioscaffolding sliced into 100 μm sections placed into a cell-well of a 96-well microtiter plate, and seeded with stem cells, 3D reconstruction of confocal scanning images and distribution of neural progenitors labeled in green and blue, from 100 μm cryogel bioscaffold sections seeded with stem cells (reproduced with permission from Jurga, M., Dainiak, M. B., Sarnowska, A., Jablonska, A., Tripathi, A., Plieva, F. M., Savina, I. N., Strojek, L., Jungvid, H., Kumar, A., Lukomska, B., Domanska-Janik, K., Forraz, N., McGuckin, C. P. (2011). The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials, 32, 3423–3434); (c) evidence of cellular attachment, proliferation, and survival, on gelatin-pendant methacrylate cryogel bioscaffolds in vitro: Representative 2-photon microscopy image of labeled cells at a depth of 150 μm below the surface of rhodamine-gelatin cryogel 2 hr after cell-seeding, SEM image of cells attached onto cryogel surface 1 day postcell-seeding; cells are false-colored for emphasis, staining for F-actin on histological sections of cell-seeded cryogels demonstrating cell-spreading within bioscaffolds 1 day postcell-culturing, and staining for visualization of de novo DNA synthesis within cryogel bioscaffold (reproduced with permission from Koshy, S. T., Ferrante, T. C., Lewin, S. A., & Mooney, D. J. (2014). Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials, 35, 2477–2487)