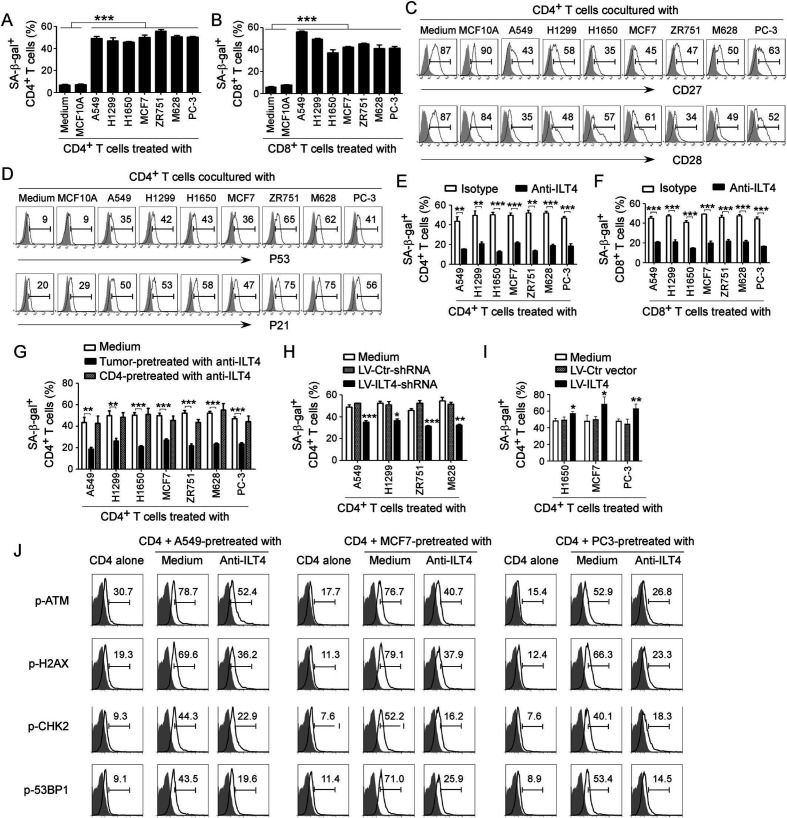
Figure 2.
Tumor-derived immunoglobulin-like transcript 4 (ILT4) is responsible for T cell senescence. (A, B) Coculture with different tumor cells significantly increased SA-β-Gal+ T cell populations in cocultured CD4+ (in A) and CD8+ (in B) T cells. However, T cells cocultured with the breast epithelial cell line MCF10A had no or little senescence-associated β-galactosidase (SA-β-Gal) expression. Anti-CD3 pre-activated naïve T cells were cocultured with tumor cells or control MCF10A cells at a ratio of 1:1 for 24 hours. Cocultured CD4+ and CD8+ T cells were then separated and cultured for additional 3 days for SA-β-Gal activity analyses. Tumor cell lines included non-small cell lung cancer (NSCLC) (A549, H1299 and H1650), breast cancer (MCF7 and ZR751), melanoma (M628) and prostate cancer (PC-3). Results shown in the histograms are mean ± SD from three independent experiments. ***p<0.001. (C, D) Tumor cell treatment decreased expression of costimulatory molecules CD27 and CD28 (in C) but upregulated expression of cell cycle regulatory molecules P53 and P21 (in D) in senescent T cells. Cell treatment and procedure were the same as in (A). Expression levels of CD27, CD28, P53, and P21 in cocultured T cells were analyzed by the flow cytometry analyses. (E, F) Blockade of ILT4 through an anti-ILT4 neutralizing antibody remarkably prevented tumor-induced senescence in both CD4+ (in E) and CD8+ (in F) T cells. The cell coculture procedures are identical to (A) and (B). Anti-ILT4 neutralizing antibody (500 ng/mL) or isotype control antibody were included in the coculture system. Results shown in the histogram are mean ± SD from three independent experiments. **p<0.01 and ***p<0.001. (G) Tumor cells but not T cells pretreated with anti-ILT4 antibody decreased SA-β-Gal+ cell populations in cocultured CD4+ T cells. Different tumor cells or anti-CD3 pre-activated naïve CD4+ T cells were pretreated with anti-ILT4 antibody (500 ng/mL) for 2 hours, and then cocultured with untreated T cells or tumor cells, respectively, as described in (A). Results shown in the histogram are mean ± SD from three independent experiments. **p<0.01 and ***p<0.001. (H) Knockdown of ILT4 in tumor cells prevented T cell senescence induced by tumor cells. Tumor cell lines (A549, H1299, ZR751 and M628) with ILT4 high expression were infected with lentivirus carrying ILT4 shRNA or control shRNA at the multiplicity of infection (MOI) of 5–10 for 48 hours and then cocultured with T cells as described in (A) and (B). SA-β-Gal+ cell populations in cocultured CD4+ T cells were determined. Results shown in the histogram are mean ± SD from three independent experiments. *p<0.05, **p<0.01, and ***p<0.001, compared with the control shRNA (LV-Ctr-shRNA) group. (I) Overexpression of ILT4 in tumor cells promoted T cell senescence induced by tumor cells. Tumor cell lines (H1650, MCF7, and PC-3) with ILT4 low expression were infected with lentivirus carrying ILT4 or control vector at the MOI of 5–10 for 48 hours and then cocultured with T cells as described in (A) and (B). SA-β-Gal+ cell populations in cocultured CD4+ T cells were determined. Results shown in the histogram are mean ± SD from three independent experiments. *p<0.05 and **p<0.01, compared with the control vector group. (J) Blockade of ILT4 prevented tumor-induced DNA damage response in CD4+ T cells. Different tumor cells were pretreated with anti-ILT4 neutralizing antibody (500 ng/mL) for 2 hours, then cocultured with anti-CD3 pre-activated naïve CD4+ T cells at the ratio of 1:1 for 24 hours. Cocultured CD4+ T cells were separated and cultured for additional 3 days. Phosphorylation levels of ATM, H2AX, CHK2, and 53BP1 in cocultured T cells were analyzed by the flow cytometry analyses.