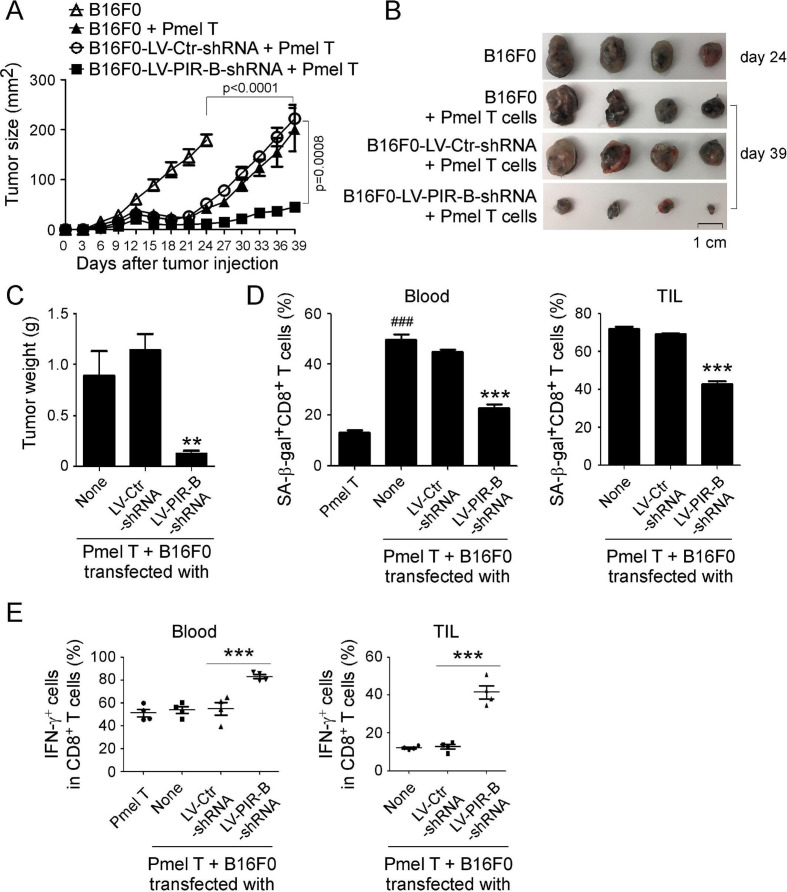
Figure 7.
PIR-B blockade enhances antitumor efficacy mediated by the adoptively transferred tumor-specific CD8+ T cells in vivo. (A–C) PIR-B knockdown showed enhanced antitumor immunity mediated by the adoptively transferred CD8+ T cells. Mouse melanoma cell line B16F0 cells (2 × 105 cells/mouse) infected with/without lentivirus carrying shRNA against PIR-B or control shRNA were subcutaneously injected into 6–8 week C57BL/6 mice. When the tumor diameter reached 5–6 mm, all the mice were intravenously injected with anti-CD3/anti-CD28 pre-activated Pmel CD8+ T cells (2 × 106 cells/mouse) after irradiation with a dose of 500 cGy. Tumors sizes were measured every 3 days (in A). When mice were euthanized, tumors in each group were isolated and weighed (in B and C). All the data are presented as mean ± SD (n=5 mice/group). **p<0.01, compared with the LV-Ctr-shRNA group (in C). (D) Knockdown of PIR-B in tumor cells significantly prevented senescence induction in the adoptively transferred CD8+ T cells in blood and TILs from tumor-bearing mice. The transferred Pmel CD8+ T cells were isolated from blood and tumor tissues, and stained for SA-β-Gal. Data shown are mean ± SD from different groups (n=4 mice/group). ***p<0.001, compared with the LV-Ctr-shRNA group. ###p<0.001, compared with the Pmel T only group. (E) Knockdown of PIR-B in tumor cells enhanced IFN-γ-producing cell populations in adoptively transferred CD8+ T cells in the blood and TILs. IFN-γ+CD8+ T cell populations in blood and TILs were determined by the flow cytometry analysis. Results shown are mean ± SD from different groups (n=4 mice/group). ***p<0.001, compared with the LV-Ctr-shRNA group.