Abstract
Immune checkpoint inhibitors (ICIs) have improved overall survival for cancer patients, however, optimal duration of ICI therapy has yet to be defined. Given ICIs were first used to treat patients with metastatic melanoma, a condition that at the time was incurable, little attention was initially paid to how much therapy would be needed for a durable response. As the early immunotherapy trials have matured past 10 years, a significant per cent of patients have demonstrated durable responses; it is now time to determine whether patients have been overtreated, and if durable remissions can still be achieved with less therapy, limiting the physical and financial toxicity associated with years of treatment. Well-designed trials are needed to identify optimal duration of therapy, and to define biomarkers to predict who would benefit from shorter courses of immunotherapy. Here, we outline key questions related to health, financial and societal toxicities of over treating with ICI and present four unique clinical trials aimed at exposing criteria for early cessation of ICI. Taken together, there is a serious liability to overtreating patients with ICI and future work is warranted to determine when it is safe to stop ICI.
Keywords: Costimulatory and Inhibitory T-Cell Receptors, Immunotherapy, Review, Melanoma
Durable responses to cancer immunotherapy
Cancer care across histologies has changed at break-neck speed in the past decade. Since the first Food and Drug Administration (FDA) approval of the Cytotoxic T-lymphocyte Antigen-4 (CTLA-4) antibody ipilimumab for metastatic melanoma a decade ago, dozens of approvals for antibodies targeting programmed cell death protein 1 (PD-1) or programmed death ligand 1 (PD-L1) alone or in combination with ipilimumab and/or chemotherapy have followed. Historically in oncology, with few exceptions, 5-year survival has been associated with ‘cure’. In the last few years—specifically in melanoma where the majority of immune checkpoint inhibitor (ICI) agents were first tested in a phase 3 setting—mature 5-year overall survival (OS) data is now being reported. In the KEYNOTE-001 clinical trial testing the safety and efficacy of pembrolizumab (anti-PD-1), there was a reported 5-year OS of 34% in all patients, with 16% of patients achieving a complete response (CR).1 In KEYNOTE-001, discontinuation was permitted in patients after receiving ≥2 pembrolizumab doses beyond the initial determination of CR and who received pembrolizumab treatment for ≥6 months. CR was confirmed by imaging scans ≥4 weeks apart and discontinuation was at the discretion of the investigator and if the patient desired. These patients were eligible to receive a second course of pembrolizumab. Seventy-two patients met criteria to discontinue therapy per protocol and entered observation. Sixty-seven achieved CR and five achieved partial response (PR) as best overall response (BOR) while seven of these patients had progressive disease post cessation (six prior CR, one prior PR). Strikingly, however, 90% of responses were maintained.1 Similarly, durable responses were seen in the KEYNOTE-006 phase 3 trial, investigating single-agent treatment of pembrolizumab or ipilimumab in 834 advanced melanoma patients.2 Nineteen per cent of patients completed the planned 2 years of pembrolizumab treatment, of which 20% achieved a BOR of CR, 67% PR and 13% had stable disease (SD).3 Responses continued after treatment cessation in 76% of CRs, 77% of PRs and 54% of SD. Remarkably, 8% of patients with previous BOR of PRs converted to CRs after cessation of pembrolizumab.3 Among patients who received 2 years of treatment of pembrolizumab and had at least SD, 78% of patients exhibited sustained disease control and remained progression free 2 years after pembrolizumab completion. The 2-year OS was 96% and the 3-year OS was 94%. Estimated 2-year progression free survival (PFS) was 85% for CR, 82% for PR and 40% for SD. Importantly, 23 patients with CRs who stopped pembrolizumab treatment earlier than 2 years, as allowed by the protocol, exhibited a PFS rate of 86%, similar to CRs who completed the full 2-year regimen. As one may expect, at the end of 2 years, patients with SD progressed more quickly than those with CR or PR. Of the patients taken off pembrolizumab, 74% remained progression free, while 26% had progressive disease. Of those patients that progressed, 44% received a second course of pembrolizumab, and more than half were again able to achieve a response.3 Further evidence for stable responses in patients with metastatic melanoma after early discontinuation of ICI was shown in an analysis of a real-world cohort. Of 52 patients who electively discontinued PD-1 inhibitors after 1 year (>6 months and <18 months) in the setting of ongoing treatment response or disease stability, after median follow-up of 20.5 months (range 3–49.2) from treatment discontinuation, 39 (75%) patients remained without disease progression (median PFS not reached).4
While treatment discontinuation due to adverse events makes outcome comparison challenging, evidence from the Keynote-006 trial suggests that some patients may continue to derive benefit from ICI therapy after discontinuation, suggesting durable benefits may be feasible from shorter courses of therapy than defined by current protocols. In a pooled analysis of randomized phase 1 and phase 2 trials of combination nivolumab and ipilimumab, efficacy outcomes were found to be comparable between patients who discontinued treatment due to immune-related adverse events (irAEs) during the early phase of the trial, and those who did not.5 Here, the proportion of CRs, and the time to response, were approximately equal in the discontinued group and the continuation group. On reanalysis of the same data, Horiguchi et al further concluded that patients in the discontinuation group were in fact predicted to live longer than those in the continued treatment group, lending credence to the notion that patients experiencing irAEs during immunotherapy may be those in which a strong immune response has been induced.6 Similarly, long-term responses to ipilimumab can be achieved after discontinuation due to irAE even after short treatment durations.7
Evidence from these early pembrolizumab trials in melanoma reflects data from nivolumab and combination nivolumab–ipilimumab trials,8 as well as real-world data on patients who cease therapy due to toxicity or patient preference. These data demonstrate that patients can experience durable responses with low incidence of relapse after significantly shorter treatment times than are mandated by trial design.9–11 The likelihood of an individual patient experiencing a sustained response after a relatively short time on treatment is likely to depend on several factors. While biomarkers to identify patients who will achieve a durable response are lacking, there are significant data demonstrating a correlation between depth and duration of response. In one real-world analysis of patients who discontinued therapy in the absence of disease progression or treatment limiting toxicity, 14% of CRs experienced progressive disease during follow-up, as compared with 32% and 50% of partial responders and patients with SD, respectively.12 Another single institution series observed that among 102 patients that achieved CR to anti-PD-1 therapy who discontinued treatment after a median treatment time of 9.4 months, 72% remained alive at 3-year follow-up without further treatment.13 Smaller studies have provided further anecdotal evidence of this pattern, with partial responders experiencing longer PFS after treatment discontinuation than patients with SD.14 15 Collectively, this suggests that among complete responders, risk of relapse after discontinuation is low even after treatment for only 6 months, though this data also demonstrate that a significant number of patients who achieve only radiographic PR or even SD may derive long-term benefit from shorter periods to treatment. Studies specifically designed to investigate duration of therapy, and biomarkers of durable responses are required to establish optimal treatment durations for those patients with PR or SD.
As data from trials across histologies mature, and with increased real-world experience, clinicians and patients achieving prolonged benefit from ICI are increasingly being faced with the dilemma of whether or not to proceed according to the design of trials that led to FDA approval, as has been the standard of care, or to risk discontinuing a successful therapy. Based on the collective experience with maintenance chemotherapy, and our understanding that metastatic cancer is nearly always a terminal illness, early trials in melanoma which specified either 2 years or indefinite therapy were followed by a large number of registrational studies in a variety of other cancers (table 1). These trials have perpetuated what is now considered a standard trial design of prolonged maintenance therapy, despite the data from melanoma trials suggesting that this may constitute overtreatment. Indeed, while early trials treated indefinitely, and the majority of trials today treat for 2 years, the benefit of ICI is typically seen very early, potentially even within the first week.16 These neoadjuvant trials in which patients have received relatively brief courses of therapy ahead of surgery have countered the belief that response to immunotherapy is slow, though radiographic responses may be delayed due to inability to differentiate a robust immune response (and subsequent radiographic scar formation) from progressive disease. If there is a vaccinal effect on lymphoid memory, one could hypothesize that only short treatments are needed, akin to the comparatively brief treatments needed with IL-2 to induce durable remissions.17 However, one retrospective analysis of a large cohort of patients who had achieved a CR did find an association between recurrence and ICI treatment of less than 6 months.12 In summary, early data from retrospective cohorts and pooled/subgroup analysis from clinical trials suggest that certain subsets of patients, particularly patients with durable response or irAEs, might benefit from cessation of immunotherapy, yet additional work will be needed for clinical utility. Prospective studies with elective discontinuation design are warranted to further elucidate the timing and indication for immunotherapy discontinuation, and additionally trials must assess the impact on OS—specifically addressing whether it is safe, and potentially non-inferior, to hold therapy and restart if/when a patient’s disease progresses.
Table 1.
Approvals for the use of immune checkpoint inhibitors
| Target | Agent | Indication | FDA approval based on registrational trial |
| PD-1 | Pembrolizumab | Colorectal cancer—microsatellite instable | Until progression, unacceptable toxicity, or up to 24 months |
| Cutaneous squamous cell carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Endometrial carcinoma* | Until progression, unacceptable toxicity, or up to 24 months | ||
| Esophageal carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Gastric carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Head and neck squamous cell carcinoma* | Until progression, unacceptable toxicity, or up to 24 months | ||
| Hepatocellular carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Hodgkin’s lymphoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Malignant pleural mesothelioma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Melanoma† | Until progression or unacceptable toxicity | ||
| Merkel cell carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Microsatellite instable cancers (histology agnostic) | Until progression, unacceptable toxicity, or up to 24 months | ||
| Non-small cell lung carcinoma* | Until progression, unacceptable toxicity, or up to 24 months | ||
| Primary mediastinal B-cell lymphoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Renal cell carcinoma* | Until progression, unacceptable toxicity, or up to 24 months | ||
| Small cell lung carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Urothelial carcinoma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Nivolumab | Colorectal cancer - microsatellite instable* | Until progression or unacceptable toxicity | |
| Esophageal carcinoma | Until progression or unacceptable toxicity | ||
| Head and neck squamous cell carcinoma* | Until progression or unacceptable toxicity | ||
| Hepatocellular carcinoma* | Until progression or unacceptable toxicity | ||
| Hodgkin’s lymphoma | Until progression or unacceptable toxicity | ||
| Melanoma*† | Until progression or unacceptable toxicity | ||
| Malignant pleural mesothelioma | Until progression, unacceptable toxicity, or up to 24 months | ||
| Non-small cell lung carcinoma* | Until progression or unacceptable toxicity | ||
| Renal cell carcinoma* | Until progression or unacceptable toxicity | ||
| Small cell lung carcinoma* | Until progression or unacceptable toxicity | ||
| Urothelial carcinoma | Until progression or unacceptable toxicity | ||
| Cemiplimab | Cutaneous squamous cell carcinoma | Until progression or unacceptable toxicity | |
| PD-L1 | Atezolizumab | Breast cancer, triple negative* | Until progression or unacceptable toxicity |
| Hepatocellular carcinoma* | Until progression or unacceptable toxicity | ||
| Melanoma* | Until progression or unacceptable toxicity | ||
| Non-small cell lung carcinoma* | Until progression or unacceptable toxicity | ||
| Small cell lung carcinoma* | Until progression or unacceptable toxicity | ||
| Urothelial carcinoma | Until progression or unacceptable toxicity | ||
| Durvalumab | Non-small cell lung carcinoma (Stage III) | Following chemoradiation, until progression, unacceptable toxicity, or up to 12 months | |
| Small cell lung carcinoma* | Until progression or unacceptable toxicity | ||
| Urothelial carcinoma | Until progression or unacceptable toxicity | ||
| Avelumab | Gestational trophoblastic neoplasia | Until progression or unacceptable toxicity, of for three cycles after normalization of hCG | |
| Merkel cell carcinoma | Until progression or unacceptable toxicity | ||
| Renal cell carcinoma* | Until progression or unacceptable toxicity | ||
| Urothelial carcinoma | Until progression or unacceptable toxicity |
*Approval includes combination therapies.
†Approval also exists in the adjuvant setting for 12 months.
FDA, Food and Drug Administration; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1.
Physical toxicity from prolonged immunotherapy
Immunotherapy treatments generally have reduced high-grade toxicities compared with conventional cytotoxic chemotherapy.2 18 Despite this, it is not uncommon for patients to experience irAEs, most frequently involving the skin, gastrointestinal tract, lung and endocrine glands but also potentially manifest as neurologic, hepatic, rheumatological, renal and cardiac toxicities.19 20 Although most irAEs are of mild or moderate severity (grades 1 and 2), clinically significant grade 3 or 4 irAEs have been reported in up to 20% of patients with single-agent PD-1 immunotherapy.20 Grade 3 or 4 irAEs are even more prevalent in patients treated with combination treatment targeting both PD-1 and CTLA-4, affecting 59% of patients.21 These side effects frequently result in interruptions of immunotherapy treatment and require immunosuppressants such as corticosteroids which themselves carry the risk of toxicities and may be associated with poorer survival outcomes when used long-term.22 Biological immunomodulatory agents may be required to manage patients with severe or life threatening irAEs, which can result in permanent treatment discontinuation and/or significant and long-term patient morbidity, if not death (figure 1).23
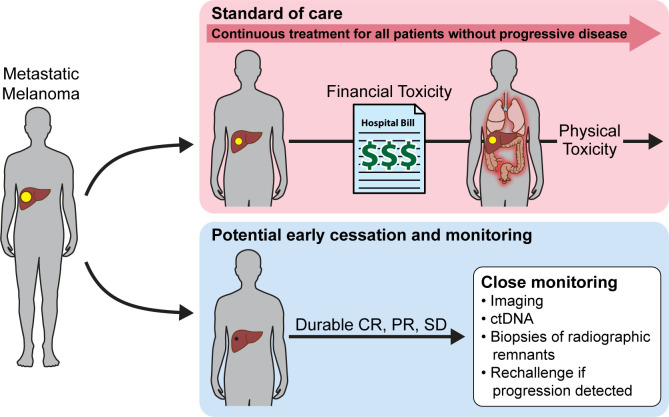
Figure 1.
Patients receiving immune checkpoint inhibitors without progressive disease are treated for an undefined period, which can extend several years and may impose both financial and physical toxicity. Clinical trials are needed to determine criteria that would allow potential early cessation and monitoring thus eliminating both financial and physical toxicities. CR, complete response; ctDNA, circulating tumor DNA; PR, partial response; SD, stable disease.
Due to the heterogeneous nature and time to onset of these toxicities, early identification of irAE symptoms and timely intervention can be challenging. Although a majority of irAEs occur within the first 5–15 weeks, there are reports of late-onset toxicities both in the setting of ongoing immunotherapy and after treatment cessation.24–27 These delayed irAEs have been reported to occur months to years after discontinuation of therapy.28 29 In a recent study, approximately half of the delayed irAE cases were observed among patients who had previously reported on-treatment irAEs, which in many cases were the reason for cessation of therapy.29 However, this study found only 21 cases of delayed irAE in response to checkpoint blockade immunotherapy from a decade of literature, suggesting a low prevalence of serious irAEs after discontinuation of therapy.29 While these delayed irAE are more rare than early-onset toxicities they do occur and can be severe; in one small retrospective study looking at 325 patients treated with ICI, within the 12% of patients continuing therapy for more than 1 year, 15% developed delayed irAEs.30 Case reports periodically highlight late onset irAEs, yet in the absence of a consistent reporting system, knowledge-based decisions on optimal duration of checkpoint inhibitor therapy in responsive patients is challenging.28 Regardless, it’s important to note that prolonged use of ICI comes with physical risk.
Financial toxicity from prolonged treatment with immunotherapy
Although ICIs have undoubtedly made an impact on the survival of patients, they have also imposed a significant financial burden on patients, their families, as well the public and private healthcare industry. As a consequence of durable remissions with limited understanding of the optimal duration of ICI treatment, patients often remain on immunotherapy for prolonged periods of time (up to several years). This has a significant financial impact on both the patient and the healthcare system (figure 1).
A recent National Cancer Institute study based on retrospective Surveillance, Epidemiology and End Results registry data estimated the annual expense of cancer care in the United States to be over US$200 billion in 2020, and projected that this will approach US$250 billion by 2030.31 Given the rapid pace of development of novel cancer therapies, these projections may significantly underestimate true societal expenses. While the prolonged survival—and potential cure—that is being realized with cancer immunotherapy is a phenomenal step forward for the field of oncology, the rapid rise in expense must be promptly addressed to ensure continued access to these agents, the development of new immunotherapies to further improve on the current state of cancer care, and continued scientific progress.
Along with the societal financial toxicity, the increase in drug costs encourages for-profit insurance providers to place greater financial responsibility on the patient in the form of increased deductibles, copays and premiums, making the associated personal and societal financial toxicities unavoidable.32 While cancer-associated costs will be specific to each patient depending on the diagnosis, treatment type and level of insurance coverage, many patients report the need for assistance in budgeting, understanding their coverage, and seeking financial help due to nuances of personalized therapy.
With treatment costs for cancer immunotherapy often in excess of US$100 000 per year, even those with a standard employer-sponsored health plan with 20% coinsurance are faced with bills that exceed 50% of the average US household income. The combination of nivolumab and ipilimumab for a typical patient has been estimated to cost US$295 566, an out-of-pocket cost of US$60 000 annually.33 As many as 40% of patients have been found to experience difficulties paying medical bills, with up to 11% missing recommended treatments, 12% lowering the dose of prescription medications, and 12% missing additional appointments or follow-up testing to reduce overall costs.34 In addition to the implications for treatment discontinuation or refusal, cancer patients are over 2.5 times more likely to declare bankruptcy as compared with healthy adults,35 and financial difficulties related to cancer care are a risk factor for mortality.36 The out-of-pocket cost of cancer immunotherapy to the individual patient, as well as the cost that long-term treatment exacts on a family and caregivers due to missed work and lost income, serves as additional motivation for identifying the optimal duration of treatment.
Trials investigating cessation of ICI therapy
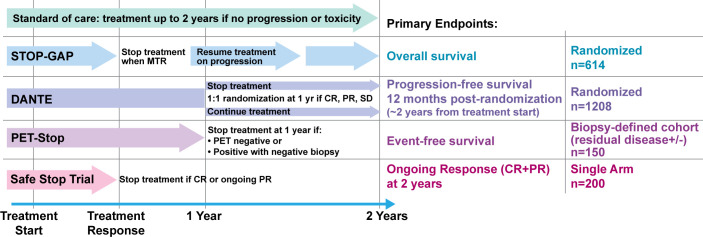
Registrational phase 3 trials have been insufficiently powered to determine an adequate duration to maintain ICI therapy, nor the potential safety hazards of relinquishing treatment. A single industry-sponsored trial in non-small-cell lung cancer (NSCLC) has evaluated the potential to stop immunotherapy early, and confirmed the need for continued treatment, though the design of the trial to include patients without mandating SD and durable disease response has drawn criticism.37 With significant data on long-term follow-up from the initial ICI trials, melanoma is the ideal clinical space to test whether to stop treatment early. In the past year, several trials have opened, all sponsored by government backed healthcare organizations, which are aimed to define optimal duration (figure 2). The DANTE (Detection And screening of early lung cancer with Novel imaging TEchnology) trial (ISRCTN15837212), for example, is a randomized phase 3 trial designed to evaluate feasibility of stopping first-line anti-PD-1 monotherapy (nivolumab or pembrolizumab) at 12 months in patients who are progression-free. Led by the UK National Cancer Research Institute Skin Cancer Clinical Studies Group, DANTE randomizes patients with unresectable stage III or stage IV melanoma who have received 1 year of PD-1 blockade without having their disease progress to either (1) stopping treatment (with the option to restart anti-PD-1 therapy or commence other treatment on progression) or (2) continuing treatment for another year or until disease progression/unacceptable toxicity. This is a non-inferiority trial that will enroll 1208 patients, and the primary outcome is PFS 1 year from randomization (2 years from initiation of immunotherapy), while secondary outcomes include quality of life assessment, OS, response rate and both physical and financial toxicities. Its notable that the investigators are also assessing cost-effectiveness, and they are also using multiple standardized quality of life metrics including the validated quality of life questionnaires (QLQs) European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30, QLQ-MEL38 and the EQ-5D-5L questionnaires for up to 18 months following randomization; while the trial is defined to assess non-inferiority in terms of PFS, effects on various physical and financial aspects affecting quality of life are potentially of equal importance.
Figure 2.
Ongoing prospective clinical trials to determine criteria for early discontinuation of immune checkpoint inhibitors. CR, complete response; MTR, maximal tumor response; PR, partial response; SD, stable disease.
Other trials are exploring stopping at even earlier time points based on treatment response without a minimum prespecified treatment duration, and also looking at ability to subsequently rechallenge. The Safe Stop trial (NTR7502, EudraCT: 2018-001384-23) is a study in the Netherlands looking at early discontinuation of first line anti-PD-1 therapy (nivolumab or pembrolizumab) for advanced or metastatic melanoma patients who have achieved a confirmed CR (with an interval confirmatory imaging of at least 6 weeks after first documentation) or an ongoing PR (with an interval of 12 weeks after first documentation). This single arm study will prospectively assess rates of ongoing responses in 200 patients at 2 years as its primary endpoint, with secondary endpoints evaluating duration of response, PFS, rate of anti-PD-1 rechallenge on progression and associated response and survival metrics, and OS. Associated studies (Safe Stop-QoL) will also measure quality of life, patient work productivity and impact on caregivers, which will help address key survivorship questions for this population in which many patients are considered cured of their disease. Like Safe Stop, the Canadian Clinical Trials Group study STOP-GAP (NCT02821013) is assessing the potential to stop treatment after maximal tumor response (MTR), which is determined by at least two radiologic measurements 3 months apart. A total of 614 patients with unresectable stage III or stage IV melanoma are being randomized 1:1 in this phase 3 trial to either standard 2 years of therapy in the absence of disease progression, or treatment until MTR with retreatment at the time of progression. The primary endpoint is OS, with secondary endpoints measuring PFS, objective response rate, AE rate, health-related quality of life and economic analysis. Notably, STOP-GAP is unique as compared with DANTE or Safe Stop in that its principal emphasis is on the role of re-challenge and impact of a ‘stop and go’ as opposed to continuous approach on OS, rather than the specific question of optimal initial duration of treatment.
There is a growing appreciation that imaging criteria for response as used in DANTE, Safe Stop and STOP-GAP only partially capture pathologic response, as some patients have persistent lesions radiographically without viable cancer. PET-Stop (EA6192; NCT04462406) is an ECOG/ACRIN cooperative group-led study that will address this in its biomarker-driven trial on early discontinuation of anti-PD-1 therapy in stage IIIB and stage IV melanoma. After 1 year of immune checkpoint blockade (pembrolizumab or nivolumab±ipilimumab), patients will receive a positron emission tomography (PET) scan to qualify for trial enrolment. Patients will have their immunotherapy held if their PET scan is negative or if it is positive but with subsequent negative biopsy of remaining hypermetabolic lesions (Arm A). The primary endpoint for this trial will assess event free survival after 1 year on study (ie, 2 years from starting ICI). Patients enrolled who have a positive PET scan with a biopsy showing viable cancer or inability to perform biopsy will enroll onto Arm B and be followed with serial imaging and repeat biopsy at conclusion of addition 1 year of anti-PD-1 therapy. Secondary endpoints include conversion of Arm B to pathological CR, OS, extended duration of therapy beyond 2 years total and toxicities.
Given the heterogeneity of both tumor and host biology, future strategies will need to explore a personalized treatment approach. It will be integral to obtain adequate tumor biopsies and optimal the correct blood samples in clinical trials to achieve deeper understanding of patient-specific tumor molecular features, baseline intratumoral immune and stromal environment, and both local and systemic immune responses will be critical to identifying patients likely to respond to therapy and maintain a durable response, as well as those who may be pre-disposed to toxicity. The translational tissue collection in PET-Stop will serve as an important resource for these exploratory investigations. Tumor tissue and peripheral blood collections are planned for this purpose as part of PET-Stop; gene expression in baseline tumor biopsies will characterize the preliminary immune infiltration, and serial blood analysis of circulating tumor DNA (ctDNA) and lymphoid and myeloid composition by mass cytometry (cytometry by time of flight; CyTOF) will be performed to assess for predictive and/or prognostic utility. Given emerging data on the utility of ctDNA to identify melanoma patients with deep responses to targeted and immunotherapy,38–40 it is critical to use this biomarker, potentially in addition to immune signatures in the peripheral blood in prospective trials, to confirm the predictive and prognostic utility of these technologies.
To our knowledge, early cessation of ICI is only currently being prospectively investigated in in the unresectable/metastatic melanoma setting, due to the high rates of durable response seen in these patients and extensive long-term follow-up from early trials in melanoma. Given the expanded use of ICI in the neoadjuvant/adjuvant setting—where the needed treatment period is also undefined41–43—and maturing 5-year data on the use of ICI in other histologies such as NSCLC and renal cell carcinoma,44–46 where there are also durable responses seen, many subsequent trials will be needed in these alternate settings to define optimal duration of therapy, in particular given the astronomical cost associated with these therapies being given to patients, some of whom may not even need adjuvant therapy.47 Future trials should continue to build off this framework to ultimately lead biomarker driven trials that can establish in which patients we can safely discontinue ICI therapy to spare the patient, and the healthcare system, significant toxicities.
Acknowledgments
The authors would like to express appreciation for all the members of the SPARKATHON 2018 and 2017 program, the SITC Executive Committee for their guidance, the anonymous donor of the SITC SPARKATHON program for their generous support, Dr. Anita Giobbie-Hurder for thoughtful discussions and the SITCure Advisory Panel: Drs. Lisa Butterfield, Paolo Ascierto, Daniel Chen, James Gulley, David Kaufman, and Kim Margolin.
Footnotes
Twitter: @aideenryan1, @toddbart001, @immunegirl, @DanielOlsonMD, @thenasheffect, @VaiaFlorou, @Dr_Aggen, @JennGuerriero
Collaborators: SITC 2018 Sparkathon Team SITCure.
Contributors: TM, AER, SMR, SK, RHY, DT, JZ, JET and JG: made substantial contributions to the conception and design of the review as well as drafting and revising the content. TM, TB and DHA generated the table. TM, SMR and JG generated the figures. All authors contributed to writing the review and editing. TM and JG provided overall leadership.
Funding: The authors wish to acknowledge the anonymous donor of the SITC SPARKATHON program for their generous support.
Competing interests: TM is a consultant for AstraZeneca, Genentech, Boehringe Ingelheim, Atara, and Regeneron. And receives research grant support from Bristol Myers Squibb, Regeneron, Merck, and Boehringer Ingelheim. AER receives sponsored research support from Janssen, AbbVie, Bristol Myers Squibb, Celgene SMR receives consulting fees from Eli Lilly, Daiichi Sankyo and Silverback Therapeutics and research support from Cancer Prevention and Research Institute of Texas RR190020. MER-R receives research grant support from ROCHE and Highlight Therapeutics. SC and CF are employees and shareholder at NanoString Technologies. DHA has received royalties from the University of Illinois and has received consulting fees from Boehringer Ingelheim. JG is a consultant for Glaxo-Smith Kline (GSK), Array BioPharma, Codagenix, Verseau Therapeutics and Kymera and receives sponsored research support from GSK, Eli Lilly and Array BioPharma.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Hamid O, Robert C, Daud A, et al. Five-Year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8. 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1239–51. 10.1016/S1470-2045(19)30388-2 [DOI] [PubMed] [Google Scholar]
- 4.Pokorny R, McPherson JP, Grossmann KF, et al. Clinical outcomes with early-elective discontinuation of PD-1 inhibitors (PDI) at one year in patients (PTS) with metastatic melanoma (Mm). Journal of Clinical Oncology 2020;38:10048. 10.1200/JCO.2020.38.15_suppl.10048 [DOI] [Google Scholar]
- 5.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horiguchi M, Uno H, Wei L-J. Patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab as a result of adverse events lived significantly longer than patients who continued treatment. J Clin Oncol 2018;36:720–1. 10.1200/JCO.2017.76.0983 [DOI] [PubMed] [Google Scholar]
- 7.Dick J, Enk A, Hassel JC. Long-Lasting responses under treatment with ipilimumab: an argument against maintenance therapy? Dermatology 2015;230:8–10. 10.1159/000365078 [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 9.Iivanainen S, Koivunen JP. Early PD-1 therapy discontinuation in responding metastatic cancer patients. Oncology 2019;96:125–31. 10.1159/000493193 [DOI] [PubMed] [Google Scholar]
- 10.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol 2019;30:1154–61. 10.1093/annonc/mdz110 [DOI] [PubMed] [Google Scholar]
- 13.Betof Warner A, Palmer JS, Shoushtari AN, et al. Long-Term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol 2020;38:1655–63. 10.1200/JCO.19.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard-Tessier A, Baldini C, Martin P, et al. Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer 2018;101:160–4. 10.1016/j.ejca.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 15.Ladwa R, Atkinson V. The cessation of anti-PD-1 antibodies of complete responders in metastatic melanoma. Melanoma Res 2017;27:168–70. 10.1097/CMR.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 16.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019;25:454–61. 10.1038/s41591-019-0357-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins MB, Kunkel L, Sznol M, et al. High-Dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6:S11. [PubMed] [Google Scholar]
- 18.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PubMed] [Google Scholar]
- 19.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 20.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan EY, Merl MY, Lin K. The impact of corticosteroid use during anti-PD1 treatment. J Oncol Pharm Pract 2020;26:814–22. 10.1177/1078155219872786 [DOI] [PubMed] [Google Scholar]
- 23.Michot JM, Bigenwald C, Champiat S, et al. Immune-Related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 24.Parakh S, Cebon J, Klein O. Delayed autoimmune toxicity occurring several months after cessation of anti-PD-1 therapy. Oncologist 2018;23:849–51. 10.1634/theoncologist.2017-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi S, Morimoto R, Okumura T, et al. Late-Onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol 2018;34:812.e1–3. 10.1016/j.cjca.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 26.Diamantopoulos PT, Gaggadi M, Kassi E, et al. Late-Onset nivolumab-mediated pneumonitis in a patient with melanoma and multiple immune-related adverse events. Melanoma Res 2017;27:391–5. 10.1097/CMR.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 27.Wang LL, Patel G, Chiesa-Fuxench ZC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol 2018;154:1057–61. 10.1001/jamadermatol.2018.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, von Itzstein MS, Lu R, et al. Late-Onset immunotherapy toxicity and delayed autoantibody changes: checkpoint inhibitor-induced Raynaud's-Like phenomenon. Oncologist 2020;25:e753–7. 10.1634/theoncologist.2019-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (dire) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer 2019;7:165. 10.1186/s40425-019-0645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah NJ, Ma BT, Kelly WJ, et al. Delayed toxicities with anti-PD-1 and anti-PDL-1 immune checkpoint inhibitors (ICIs). Journal of Clinical Oncology 2018;36:e15074. 10.1200/JCO.2018.36.15_suppl.e15074 [DOI] [Google Scholar]
- 31.Mariotto AB, Enewold L, Zhao J, et al. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev 2020;29:1304–12. 10.1158/1055-9965.EPI-19-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med 2018;6:166. 10.21037/atm.2018.03.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. Am Health Drug Benefits 2015;8:9. [PMC free article] [PubMed] [Google Scholar]
- 34.King D. Financial toxicity: Unspoken side effect of cancer treatment, 2017. Available: https://www.asbestos.com/blog/2017/07/25/financial-toxicity-side-effect-cancer/
- 35.Ramsey S, Blough D, Kirchhoff A, et al. Washington state cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff 2013;32:1143–52. 10.1377/hlthaff.2012.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial Insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 2016;34:980–6. 10.1200/JCO.2015.64.6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spigel D. CheckMate 153: randomized results of continuous vs 1-year fixed-duration nivolumab in patients with advanced non-small-cell lung cancer. Ann Oncol 2017;28:v460–96. [Google Scholar]
- 38.Forschner A, Battke F, Hadaschik D, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J Immunother Cancer 2019;7:180. 10.1186/s40425-019-0659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hont AB, Cruz CR, Ulrey R, et al. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor associated antigen specific cytotoxic T lymphocytes: a phase I study. J Clin Oncol 2019;37:2349–59. 10.1200/JCO.19.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205–11. 10.1200/JCO.2013.49.8691 [DOI] [PubMed] [Google Scholar]
- 41.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 42.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35. 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 43.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol 2019;20:948–60. 10.1016/S1470-2045(19)30151-2 [DOI] [PubMed] [Google Scholar]
- 44.Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019;37:2518–27. 10.1200/JCO.19.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or Non–Small cell lung cancer treated with nivolumab. JAMA Oncol 2019;5:1411–20. 10.1001/jamaoncol.2019.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563–73. 10.1016/S1470-2045(20)30436-8 [DOI] [PubMed] [Google Scholar]
- 47.Stav I, Gyawali B, Goldstein DA. Duration of adjuvant immunotherapy-biologic, clinical and economic considerations. Med Oncol 2018;35): :160. 10.1007/s12032-018-1218-0 [DOI] [PubMed] [Google Scholar]