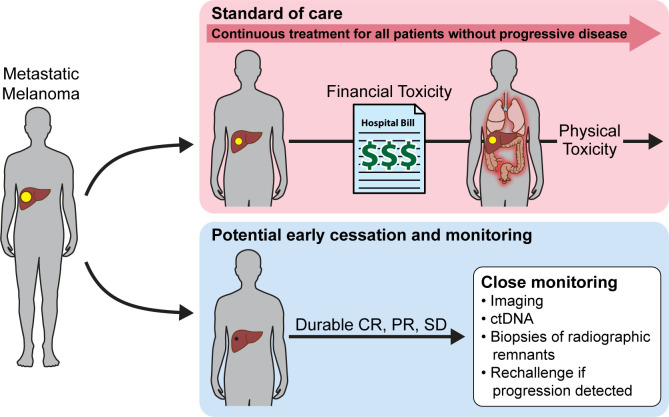
Figure 1.
Patients receiving immune checkpoint inhibitors without progressive disease are treated for an undefined period, which can extend several years and may impose both financial and physical toxicity. Clinical trials are needed to determine criteria that would allow potential early cessation and monitoring thus eliminating both financial and physical toxicities. CR, complete response; ctDNA, circulating tumor DNA; PR, partial response; SD, stable disease.