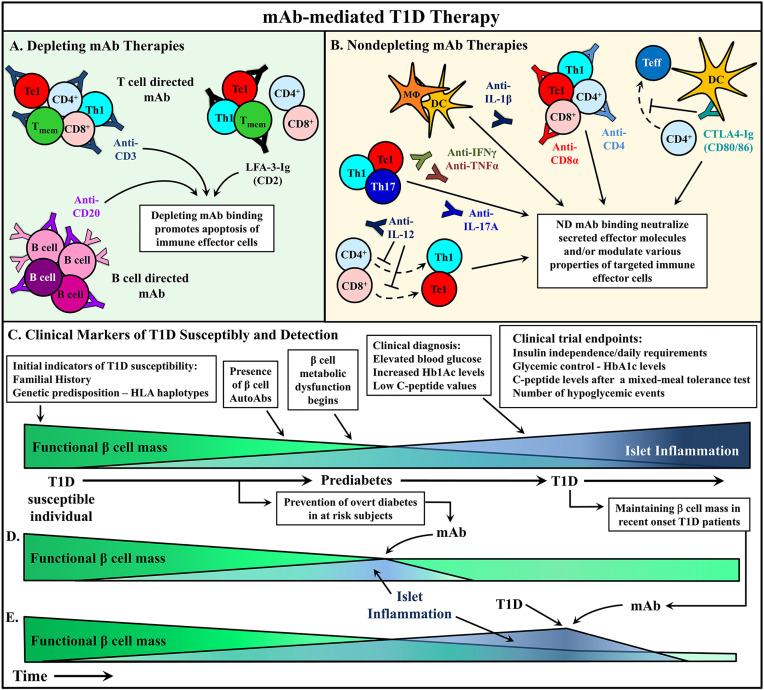
Figure 2.
mAb therapies to ameliorate type 1 diabetes (T1D). (A, B) Monoclonal antibodies (mAb) treatments can be broadly divided into two categories: depleting mAb or nondepleting (ND)/neutralizing mAb. Some promising mAb treatments are depicted that have been used in either animal models or clinical trials to alter T1D progression. (A) Depleting mAb have been used to target T and B cells in the clinic. Transient depletion of T and B cells delays the progression of β cell autoimmunity. (B) ND mAb have been applied to neutralize cytokines to suppress the proinflammatory milieu of the pancreatic lymph node (PLN) and islets, as well as modulate the properties and activity of various immune effector cells. (C) The relationship between functional β cell mass versus islet inflammation is characterized. Over time, increased chronic islet inflammation results in decreased functional β cell mass, first detected via metabolic abnormalities, and ultimately leading to deficient insulin production, prompting clinical diagnosis of T1D. Individuals at different stages of T1D progression have treated with mAb therapies to alter T1D progression, and in turn (D) prevent diabetes onset, or (E) rescue residual β cell mass after clinical T1D diagnosis. Typically, these clinical trials have used metabolic readouts for β cell function as primary endpoints to determine therapeutic efficacy (C).