Abstract
Objective
To compare 5-year disease-free survival (DFS) and overall survival (OS) rates of laparoscopic radical hysterectomy (LRH) and abdominal radical hysterectomy (ARH) for stage IB1 and tumor size <2 cm with visible or invisible tumors.
Methods
We retrospectively compared the oncological outcomes of 1,484 cervical cancer patients with IB1 and tumor size <2 cm on final pathology, who received ARH (n=899) or LRH (n=585) between January 2004 and December 2016. Patients were divided into visible tumor subgroup (ARH: n=668, LRH: n=444) and invisible tumor subgroup (ARH: n=231, LRH: n=141) according to tumor type.
Results
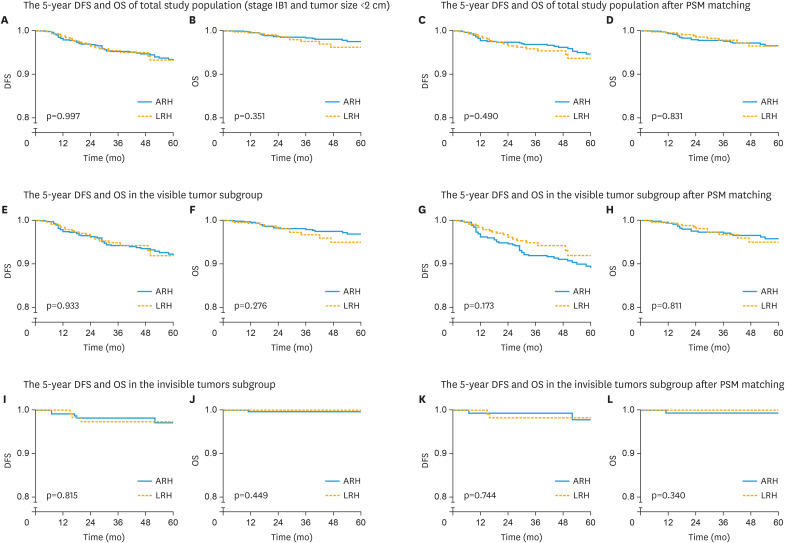
LRH and ARH showed similar 5-year DFS and OS rates (93.3% vs. 93.1%, p=0.997; 96.2% vs. 97.5%, p=0.351) in total study population. LRH was not associated with worse 5-year DFS rate (hazard ratio [HR]=0.96; 95% confidence interval [CI]=0.58–1.58; p=0.871) or OS rate (HR=1.37; 95% CI=0.65–2.89; p=0.409) by multivariable analysis. In the visible tumor subgroups, LRH and ARH showed similar 5-year DFS and OS rates (91.9% vs. 91.9%, p=0.933; 95.0% vs. 96.9%, p=0.276), and LRH was not associated with worse 5-year DFS or OS rate (p=0.804, p=0.324). In the invisible tumor subgroups, LRH and ARH also showed similar 5-year DFS and OS rates (97.3% vs. 97.1%, p=0.815; 100% vs. 99.5%, p=0.449), and LRH was not associated with worse 5-year DFS rate (p=0.723).
Conclusions
Among patients with stage IB1 and tumor size <2 cm, whether the tumor is visible or not, the oncological outcomes of LRH and ARH among cervical cancer patients are comparable. This suggests that LRH may be suitable for stage IB1 and tumor size <2 cm with visible or invisible tumors.
Trial Registration
International Clinical Trials Registry Platform Identifier: CHiCTR180017778
Keywords: Cervical Cancer, Laparoscopy, Laparotomy, Hysterectomy, Disease-Free Survival
INTRODUCTION
Since laparoscopic radical hysterectomy (LRH) was reported in 1992 [1], minimally invasive surgery (MIS) for cervical cancer has been used worldwide. Previous studies [2,3,4,5] revealed that patients with early stage cervical cancer can benefit from laparoscopic surgery, the oncology outcomes of laparoscopic and open radical hysterectomy are similar, and laparoscopic surgery has the advantages of short hospital stay, less bleeding, low blood transfusion rate, more lymph nodes removed, fast recovery and fewer postoperative complications.
However, a high-quality and international multicentre randomized controlled trial, the Laparoscopic Approach to Cervical Cancer (LACC) Trial [6], demonstrated that minimally invasive radical hysterectomy was associated with lower rates of 4.5 years disease-free survival (DFS) and overall survival (OS) than abdominal radical hysterectomy (ARH). A retrospective epidemiological study based on the National Cancer Database and the Surveillance, Epidemiology, and End Results database also reached similar conclusions [7]. Subsequently, several retrospective cohort studies from other countries demonstrated that minimally invasive radical hysterectomy was associated with worse oncological outcomes than open radical hysterectomy among patients with early stage cervical cancer [8,9]. Based on these clinical evidences, open surgery has been recommended as the only standard approach for radical hysterectomy starting with Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology [10].
Meanwhile, several studies suggest that MIS has similar oncological outcomes as open surgery in cervical cancer patients with stage IB1 and tumor size <2 cm [7,9]. However, articles suggestive of no difference in outcomes in patients with tumors <2 cm often are hindered by low volume of patients in such group and are not powered nor designed to demonstrate a difference. There were also some studies have come to the opposite conclusion [11,12]. Whether MIS is suitable for stage IB1 cervical cancer with tumor size <2 cm is still controversial. In addition, there has not been further analysis for patients with stage IB1and tumor size <2 cm with visible or invisible tumors.
Therefore, based on the clinical diagnosis and treatment for cervical cancer in mainland China (Four C) database, this study aimed to compare the 5-year OS and DFS of ARH and LRH in patients with stage IB1and tumor size <2 cm with visible or invisible tumors.
MATERIALS AND METHODS
1. Data source
This study was a multicentre, retrospective, observational study, and the data used in this study originated from the Four C database, a cervical cancer specialized disease database (n=46 313) that covers consecutive patients with cervical cancer in 37 hospitals in mainland China treated between January 2004 and December 2016. The establishment of the cervical cancer database was reviewed by the Ethics Committee of Nanfang Hospital, Southern Medical University (ethics number NFEC-2017-135) and written informed consent was waived by the Ethics Committee. The identifier of the clinical trial is CHiCTR180017778 (International Clinical Trials Registry Platform Search Port, http://apps.who.int/trialsearch/).
Clinical data were collected from patient files and the medical record management system in the hospitals by trained gynaecological oncology staff using standardized data collection and quality control procedures. The details of the data sources and methods were the same as those previously reported [13,14,15]. For patients underwent surgical treatment, the collected data contained almost all the information during the treatment of cervical cancer, including demographic details, preoperative examination results, surgical information, pathological results, preoperative and postoperative adjuvant treatment details, complications, hospitalization time and expenses, and follow-up. To ensure the accuracy of the collected data, two uniformly trained staff used EpiData software (EpiData Association, Odense M, Denmark) to input and proofread the same data from each hospital.
All follow-up procedures were carried out by trained gynaecological oncology staff at each centre to keep the patients' personal data confidential and to simultaneously provide disease management guidance. Follow-up information, including the survival status, time of death, recurrence time, recurrence site, and treatment after recurrence, was gathered through the return visit system or through a telephone follow-up. Vaginal stump recurrence was usually confirmed by pathological biopsy, abdominal and pelvic recurrence is detected by computer tomography (CT) or magnetic resonance imaging (MRI), and a few patients are detected by positron emission tomography-CT. The oncological outcomes were estimated according to the recorded information, and the last day of the return visit or telephone follow-up was defined as the last follow-up. In this database, the final International Federation of Gynecology and Obstetrics (FIGO) stage was corrected by tumor size according to the FIGO 2009 staging system. Tumor size was determined by final pathological records.
2. Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) FIGO stage IB1 (FIGO 2009 staging system) and tumor size <2 cm on postoperative pathology; 2) squamous cell carcinoma, adenocarcinoma or adenosquamous carcinoma; 3) Q-M type B or type C radical hysterectomy + pelvic lymphadenectomy ± para-abdominal aortic lymphadenectomy; and 4) laparoscopic or open surgery.
The exclusion criteria were as follows: 1) patients underwent preoperative adjuvant treatment; 2) conversion from laparoscopic surgery to open approach; 3) patients with pregnancy; 4) cervical stump cancer; 5) patients combined with other malignancies.
3. Definition
Visible tumors were defined as patients who were diagnosed with stage IB1 cervical cancer by cervical biopsy under naked eye or colposcope without cervical conization, and the tumor size measured by pathologic examination was less than 2 cm. Invisible tumors were defined as patients with no visible tumor by gynaecological examination, patients were diagnosed with stage IB1 cervical cancer by cervical conization with vertical interstitial infiltration >5 mm or horizontal infiltration >7 mm and tumor size <2 cm.
The 5-year DFS was defined as the date from the operation to the date of death due to cervical cancer or recurrence of cervical cancer. OS was defined as the date from the operation to the date of death from any cause. Patients with no evidence of recurrence or death were defined by the date of the last follow-up date or the last outpatient visit.
4. Postoperative adjuvant treatment
Patients with one or more high-risk factors (lymph node metastasis, parametrial tumor involvement, and surgical margin invasion) were recommended to receive postoperative adjuvant chemoradiation therapy. Patient with two or more intermediate-risk factors (deep cervical stromal invasion, tumor size >4 cm, and lymphovascular space invasion [LVSI]) were recommended to receive postoperative adjuvant radiation or chemoradiation therapy. In real clinical practice, there were still a small number of patients receiving chemotherapy alone. In this study, there were also some patients who received postoperative chemotherapy; we included them as an influencing variable in the multivariate analysis to minimize the impact of postoperative adjuvant treatment on the results of this study.
5. Statistical methods
Two-independent samples t-test and the χ2 test were used to analyse the clinicopathologic characteristics of the LRH and ARH groups. Kaplan-Meier curves were used to describe the survival outcomes of different surgical approaches. Cox proportional risk regression models were used to adjust for mixed cases and estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the effect of surgical approaches on the 5-year OS and DFS rates. The statistical software used was Statistical Product and Service Solutions 23.0 (SPSS, Inc., Chicago, IL, USA). The p-value <0.05 was considered statistically significant.
In the Cox proportional risk regression models, we included clinical variables regarded as known factors affecting the oncological outcomes of cervical cancer (age, histology, tumor type, depth of stromal invasion, LVSI, lymph node metastasis, surgical margin invasion, parametrial tumor involvement, and postoperative adjuvant treatment).
In the propensity score matching analysis, patients in the LRH group were matched to patients in the ARH group based on propensity score to reduce bias. Then, a new cohort of patients who differed with surgical approaches but were similar with other clinicopathological characteristics was constructed. The propensity score of each patient's propensity to undergo LRH was calculated by a logistic-regression model that included clinical variables (age, histology, tumor type, depth of stromal invasion, LVSI, lymph node metastasis, surgical margin invasion, parametrial tumor involvement, and postoperative adjuvant treatment) regarded as known factors affecting the oncological outcomes of cervical cancer. This propensity score was used for one-to-one matching cases with the nearest neighbour matching with variance of 0.02.
RESULTS
1. Study population and clinicopathologic characteristics
A total of 1484 patients met the inclusion and exclusion criteria, 585 patients in LRH group and 899 patients in ARH group (Fig. 1). The median follow-up time was 42 months in LRH group and 48 months in ARH group (p=0.521). The distribution of hospital function, region, and city scale in LRH group and ARH group was not balanced (all p>0.05), the urban-rural distribution in the 2 groups was similar, as shown in Supplementary Table 1. The clinicopathologic characteristics of the 2 groups are shown in Table 1. Patients in the LRH group were more likely to have LVSI than those in the ARH group (p=0.014). Patients in the LRH group were less likely to have no postoperative adjuvant treatment than those in the ARH group (p=0.008). The baseline differences in age, histology, infiltration depth of cervical stroma, positive parametrium, positive vaginal surgery margin, positive pelvic lymph nodes and positive para-aortic lymph nodes were not significantly different between the LRH and ARH groups (all p>0.05).
Fig. 1. Flow diagram of recruitment and exclusions.
ARH, abdominal radical hysterectomy; LRH, laparoscopic radical hysterectomy.
Table 1. The clinicopathologic characteristics of patients in LRH group and ARH group.
| Characteristics | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| ARH (n=899) | LRH (n=585) | p | ARH (n=574) | LRH (n=574) | p | ||
| Age (yr) | 46.33±9.505 | 46.86±9.423 | 0.638 | 46.29±8.836 | 46.54±9.086 | 0.640 | |
| Histologic type | 0.403 | 0.142 | |||||
| Squamous cell | 760 (84.5) | 483 (82.6) | 489 (85.2) | 473 (82.4) | |||
| Adenocarcinoma | 114 (12.7) | 88 (15.0) | 66 (11.5) | 87 (15.2) | |||
| Adenosquamous | 25 (2.8) | 14 (2.4) | 19 (3.3) | 14 (2.4) | |||
| Tumor type | 0.489 | 0.155 | |||||
| Invisible tumor | 231 (25.7) | 141 (24.1) | 157 (27.4) | 136 (23.7) | |||
| Visible tumor | 668 (74.3) | 444 (75.9) | 417 (72.6) | 438 (76.3) | |||
| Stromal invasion | 0.192 | 0.808 | |||||
| Superficial | 664 (73.9) | 410 (70.1) | 414 (72.1) | 404 (70.4) | |||
| Deep | 135 (15.0) | 108 (18.5) | 99 (17.2) | 105 (18.3) | |||
| Unreported | 100 (11.1) | 67 (11.5) | 61 (10.6) | 65 (11.3) | |||
| LVSI | 0.014 | 0.671 | |||||
| Negative | 805 (89.5) | 499 (85.3) | 496 (86.4) | 491 (85.5) | |||
| Positive | 94 (10.5) | 86 (14.7) | 78 (13.6) | 83 (14.5) | |||
| Parametrium | 0.290 | >0.999 | |||||
| Negative | 892 (99.2) | 583 (99.7) | 572 (99.7) | 572 (99.7) | |||
| Positive | 7 (0.8) | 2 (0.3) | 2 (0.3) | 2 (0.3) | |||
| Surgical margin | 0.791 | >0.999 | |||||
| Negative | 891 (99.1) | 579 (99.0) | 568 (99.0) | 568 (99.0) | |||
| Positive | 8 (0.9) | 6 (1.0) | 6 (1.0) | 6 (1.0) | |||
| Pelvic lymph nodes | 0.114 | 0.675 | |||||
| Negative | 835 (92.9) | 530 (90.6) | 526 (91.6) | 522 (90.9) | |||
| Positive | 64 (7.1) | 55 (9.4) | 48 (8.4) | 52 (9.1) | |||
| Para-aortic lymph nodes | 0.145 | 0.316 | |||||
| Negative or non-resection | 898 (99.9) | 582 (99.5) | 573 (99.8) | 571 (99.5) | |||
| Positive | 1 (0.1) | 3 (0.5) | 1 (0.2) | 3 (0.5) | |||
| Adjuvant therapy | 0.008 | 0.063 | |||||
| None | 549 (61.1) | 339 (57.9) | 342 (59.6) | 331 (57.7) | |||
| Chemotherapy | 149 (16.6) | 134 (22.9) | 102 (17.8) | 132 (23.0) | |||
| Radiotherapy/radiochemotherapy | 201 (22.4) | 112 (22.4) | 130 (22.6) | 111 (19.3) | |||
Values are presented as mean±standard deviation or number (%). Bold indicates significant p-value.
ARH, abdominal radical hysterectomy; LRH, laparoscopic radical hysterectomy; LVSI, lymphovascular space invasion.
2. DFS and OS in total study population
Among patients with stage IB1 and tumor size <2 cm, the 5-year DFS was 93.3% and 93.1% in LRH and ARH groups, respectively, with no significant difference (p=0.997), as shown in Figure 2A. Multivariable analysis controlling for demographic, socioeconomic and clinical variables indicated that surgical approaches were not an independent risk factor for worse 5-year DFS (HR=0.96; 95% CI=0.58–1.58; p=0.871, as shown in Table 2). The 5-year OS was 96.2% and 97.5% in LRH and ARH groups, respectively, with no significant difference (p=0.351), as shown in Figure 2B, and surgical approaches were not an independent risk factor for worse 5-year OS (HR=1.37; 95% CI=0.65–2.89; p=0.409, as shown in Table 2).
Fig. 2. Survival outcomes of open and laparoscopic surgery in study population and different subgroups.
ARH, abdominal radical hysterectomy; DFS, disease-free survival; LRH, laparoscopic radical hysterectomy; OS, overall survival; PSM, propensity score matching.
Table 2. Association of surgical approach and survival in cervical cancer by multivariablem analysis.
| Characteristics | Multivariate analysis for 5-year DFS | Multivariate analysis for 5-year OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Surgical approach | 0.871 | 0.409 | |||||
| Abdominal | 1 (Ref) | 1 (Ref) | |||||
| Laparoscopic | 0.96 | 0.58–1.58 | 0.871 | 1.37 | 0.65–2.89 | 0.409 | |
| Age (yr) | 1.02 | 0.99–1.04 | 0.200 | 1.06 | 1.02–1.10 | 0.001 | |
| Histologic type | 0.649 | 0.880 | |||||
| Squamous cell | 1 (Ref) | 1 (Ref) | |||||
| Adenocarcinoma | 1.05 | 0.55–2.03 | 0.878 | 0.76 | 0.26–2.23 | 0.614 | |
| Adenosquamous | 0.40 | 0.05–2.92 | 0.364 | 1.00 | 0.13–7.45 | 0.996 | |
| Tumor type | 0.001 | 0.003 | |||||
| Invisible tumor | 1 (Ref) | 1 (Ref) | |||||
| Visible tumor | 2.67 | 1.26–5.65 | 0.001 | 9.21 | 1.24–68.55 | 0.003 | |
| LVSI | 0.989 | 0.946 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 1.01 | 0.48–2.09 | 0.989 | 0.96 | 0.33–2.83 | 0.946 | |
| Stromal invasion | 0.109 | 0.222 | |||||
| Superficial | 1 (Ref) | 1 (Ref) | |||||
| Deep | 1.04 | 0.54–1.98 | 0.911 | 0.72 | 0.26–1.96 | 0.517 | |
| Unreported | 1.91 | 1.04–3.49 | 0.037 | 2.00 | 0.79–5.12 | 0.146 | |
| Parametrium | 0.707 | 0.987 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 1.50 | 0.18–12.11 | 0.707 | 0 | - | 0.987 | |
| Surgical margin | 0.959 | 0.985 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 0 | 0-2.584E+163 | 0.959 | 0 | - | 0.985 | |
| Lymph node metastasis | <0.001 | <0.001 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 3.53 | 1.84–6.76 | <0.001 | 7.19 | 2.84–18.21 | <0.001 | |
| Adjuvant therapy | 0.643 | 0.880 | |||||
| None | 1 (Ref) | 1 (Ref) | |||||
| Chemotherapy | 0.76 | 0.39–1.46 | 0.403 | 1.04 | 0.39–2.76 | 0.935 | |
| Radiochemotherapy/radiotherapy | 0.79 | 0.40–1.54 | 0.485 | 0.80 | 0.28–2.30 | 0.679 | |
Multicollinearity test and cox proportional hazard regression models were used for analysis. Proportional hazard assumption was tested and showed no interaction with time. Bold indicates significant p-value.
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LVSI, lymphovascular space invasion; OS, overall survival
After propensity score matching (n=1,148), the clinicopathologic characteristics were well balanced between the LRH and ARH groups (Table 1). LRH and ARH showed similar 5-year DFS and OS (93.7% vs. 94.3%, p=0.490; 96.5% vs. 96.5%, p=0.831; as shown in Fig. 2C and D), and surgical approaches were not an independent risk factor for worse 5-year DFS (HR=1.17; 95% CI=0.66–2.09; p=0.590) or OS (HR=0.89; 95% CI=0.41–1.92; p=0.766), as shown in Table 3.
Table 3. Association of surgical approach and survival in cervical cancer by multivariable analysis after PSM matching.
| Characteristics | Multivariate analysis for 5-year DFS | Multivariate analysis for 5-year OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Surgical approach | 0.590 | 0.766 | |||||
| Abdominal | 1 (Ref) | 1 (Ref) | |||||
| Laparoscopic | 1.17 | 0.66–2.09 | 0.590 | 0.89 | 0.41–1.92 | 0.766 | |
| Age (yr) | 1.03 | 0.10–1.06 | 0.094 | 1.06 | 1.02–1.11 | 0.002 | |
| Histologic type | 0.875 | 0.886 | |||||
| Squamous cell | 1 (Ref) | 1 (Ref) | |||||
| Adenocarcinoma | 1.09 | 0.50–2.38 | 0.825 | 0.76 | 0.26–2.26 | 0.626 | |
| Adenosquamous | 0.63 | 0.09–4.64 | 0.653 | 0.90 | 0.12–6.71 | 0.917 | |
| Tumor type | 0.010 | 0.024 | |||||
| Invisible tumor | 1 (Ref) | 1 (Ref) | |||||
| Visible tumor | 3.91 | 1.38–11.08 | 0.010 | 10.07 | 1.35–75.19 | 0.024 | |
| LVSI | 0.846 | 0.483 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 1.09 | 0.47–2.53 | 0.846 | 0 | - | 0.483 | |
| Stromal invasion | 0.147 | 0.141 | |||||
| Superficial | 1 (Ref) | 1 (Ref) | |||||
| Deep | 0.86 | 0.39–1.91 | 0.705 | 0.56 | 0.19–1.64 | 0.289 | |
| Unreported | 1.95 | 0.94–4.01 | 0.072 | 1.99 | 0.78–5.10 | 0.150 | |
| Parametrium | 0.985 | 0 | 0.987 | ||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 0 | - | 0.985 | 0 | - | 0.979 | |
| Surgical margin | 0.976 | 0 | 0.979 | ||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 0 | - | 0.976 | 0 | - | 0.977 | |
| Lymph node metastasis | 0.002 | <0.001 | |||||
| Negative | 1 (Ref) | 1 (Ref) | |||||
| Positive | 3.44 | 1.59–7.45 | 0.002 | 6.00 | 2.37–15.24 | <0.001 | |
| Adjuvant therapy | 0.769 | 0.921 | |||||
| None | 1 (Ref) | 1 (Ref) | |||||
| Chemotherapy | 0.79 | 0.37–1.70 | 0.549 | 1.90 | 0.42–2.84 | 0.862 | |
| Radiochemotherapy/radiotherapy | 0.77 | 0.33–1.80 | 0.551 | 0.87 | 0.23–2.52 | 0.791 | |
Multicollinearity test and cox proportional hazard regression models were used for analysis. Proportional hazard assumption was tested and showed no interaction with time. Bold indicates significant p-value.
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LVSI, lymphovascular space invasion; OS, overall survival; PSM, propensity score matching.
3. DFS and OS in visible tumor subgroup
Among 1112 patients with a visible tumor, 668 patients had ARH and 444 patients LRH (Supplementary Table 2). LRH showed similar 5-year DFS (91.9% vs. 91.9%, p=0.933) and OS (95.0% vs. 96.9%, p=0.276) compared to ARH (Fig. 2E and F). Surgical approaches were not an independent risk factor for worse 5-year DFS (HR=0.94; 95% CI=0.55–1.59; p=0.804) or OS (HR=1.46; 95% CI=0.69–3.11; p=0.324).
After propensity score matching, 444 patients were in each group, and the 5-year DFS (91.9% vs. 89.0%, p=0.173; HR=0.66; 95% CI=0.39–1.11; p=0.119) and OS (95.0% vs. 95.8%, p=0.811; HR=1.05; 95% CI=0.49–2.24; p=0.901) were comparable between LRH and ARH, as shown in Fig. 2G and H.
4. DFS and OS in invisible tumor subgroup
Among 372 patients with invisible tumors, 231 patients had ARH and 141 patients had LRH (Supplementary Table 3). In this subgroup, LRH showed similar 5-year DFS (97.3% vs. 97.1%, p=0.815) and OS (100.0% vs. 99.5%, p=0.449) compared to ARH (Fig. 2I and J). Surgical approaches were not an independent risk factor for worse 5-year DFS (HR=0.72; 95% CI=0.14–3.76; p=0.701). After propensity score matching, 134 patients were in each group, and the 5-year DFS (98.2% vs. 97.7%, p=0.744; HR=0.76; 95% CI=0.10–22.39; p=0.759) and OS (100.0% vs. 99.3%, p=0.340) were comparable between ARH and LRH, as shown in Fig. 2K and L.
5. DFS and OS in different tumor type
After adjusting for case mix, including age, histology, depth of stromal invasion, LVSI, lymph node metastasis, surgical margin invasion, parametrial tumor involvement, and postoperative adjuvant treatment, visible tumor was independently associated with for worse 5-year DFS (HR=2.67; 95% CI=1.26–5.65; p=0.001) and OS (HR=9.21; 95% CI=1.24–68.55; p=0.003), as shown in Table 2.
DISCUSSION
In this study, we found that the 5-year DFS and OS were similar among patients with stage IB1 cervical cancer and tumor size <2 cm on final pathology. These patients were then subdivided into visible tumor and invisible tumor subgroups. In the visible tumor subgroup, the tumor was found by gynaecological examination. In the invisible tumor subgroup, the tumor was not found by gynaecological examination; patients were diagnosed by cervical conization with vertical interstitial infiltration >5 mm or horizontal infiltration >7 mm and tumor size <2 cm on conization and final pathology. We obtained the same results in these subgroups compared to the total study population. This suggests that patients with stage IB1 and tumor size <2 cm with visible or invisible tumors can benefit from laparoscopic surgery, considering the advantages of LRH in terms of hospital stay, blood transfusion, number of lymph nodes removed, postoperative complications, and recovery time [2,3,4,5]. In the analysis of 5-year OS in the invisible tumor subgroup, we did not conduct a multivariate analysis of the associations between surgical approaches and 5-year OS because there was no death in this group.
This large multicentre retrospective cohort study complements the evidence that laparoscopic surgery may be appropriate for cervical cancer patients with stage IB1 and tumor size <2 cm. The results of this study are similar to those of several recent retrospective studies. Melamed et al. [7] found that MIS was not associated with shorter overall survival than ARH among women with stage IA2 or IB1 and tumor size <2 cm (ARH vs. MIS, 459 vs. 534). Kim et al. [9] found that open surgery and MIS had similar PFS and OS among patients with stage IB1 and ≤2 cm tumors (ARH vs. MIS, 65 vs. 24). Doo et al. [8] found that there was no difference in progression-free survival (PFS) or OS for stage IB1 and <2 cm tumors (ARH vs. LRH, 21 vs. 30). Pedone et al. [16] found laparoscopy showed DFS equivalent to ARH among IA1 with LVSI to IB1/IIA1 patient with tumor size ≤2 cm (ARH vs. LRH, 114 vs. 137).
There are also 2 studies with different results. Paik et al. [12] found LRH was associated with lower rate of DFS among patient with IB–IIA and tumor size <2 cm (ARH vs. LRH, 186 vs. 62). Uppal et al. [11] found that the MIS was associated with a higher likelihood of recurrence in the risk-adjusted analysis of IA1 with LVSI to IB1 patients with tumor size ≤2 cm (ARH vs. MIS, 82 vs. 182). In Paik's study, tumor size was determined by clinical palpation or inspection, but tumor size classification on clinical evaluation seemed to be different from the tumor size classification on final pathology, so some patients with tumor size >2 cm on final pathology may be included in the analysis. A larger percentage of MIS was robotic-assisted surgery in Uppal's study, while all of MIS was laparoscopic surgery in our study, which is a possible cause of the different results between the two studies.
Several potential reasons are regarded as contributing to the inferior oncological outcomes for LRH: uterine manipulator increasing tumor spillage, CO2 pneumoperitoneum promoting tumor cell growth or spread, and intracorporeal colpotomy increasing the risk of tumor dissemination in abdominal and pelvic cavity [6,17]. But few studies have been designed to answer this question. In this study, we analyzed patients with IB1 tumor size <2 cm, small tumor was less likely to be broken during intracorporeal colpotomy or promoted to spread by insufflation gas. We performed a subgroup analysis of visible and invisible tumor based on the hypothesis that visible tumor has a high opportunity to be squeezed and tumor spillage during the use of uterine manipulator, and found that LRH and ARH had similar 5-year OS and DFS both in visible tumor and invisible tumor subgroups. This suggests that the role of uterine manipulator may not cause poor oncological outcomes of laparoscopic surgery for patients with small tumor, and more specialized research needs to be designed to answer this question.
The strengths of our study are that large number of stage IB1 and tumors size <2 cm patients were included and further stratification analysis for patients with visible or invisible tumors was conducted. However, this study had several limitations. First, the patient files and medical records may be different among hospitals, leading to a lack of certain clinical data. Second, although the study included cervical cancer patients from 37 hospitals, it did not completely cover all institutions in mainland China. Third, there was no pathological centre, so the determination of invisible tumor was based only on the medical records of cervical conization, and the infiltration depth of cervical stroma and horizontal extension width of the lesions were not assessed by homogenization. Fourth, the FIGO 2009 staging system was adopted in this study, and the horizontal invasion width of cancer tumor >7 mm was taken as the diagnosis standard of IB1, although the horizontal invasion was cancelled in the latest FIGO 2018 staging system. The results of the invisible tumor subgroup may be affected by this change in diagnostic criteria. Fifth, we chose the pathologic tumor size as the final tumor size, when the clinical tumor size was not inconsistent with pathology. However, cervical and uterine specimens were usually cut and unfolded when measuring tumor size, resulting in pathologic tumor size tended to be larger than its actual size, especially for patients with visible tumor. It may be more appropriate to determine visible tumors by preoperative MRI, but the rate of patients received MRI was low in this database. Sixth, the data on colpotomy methods and type of uterine manipulator was not available in the database, this may limit our interpretation of the results. Seventh, this study was a retrospective study, and the oncological outcomes of 27.3% of patients in the database are unknown.
In conclusion, among cervical cancer patients with stage IB1 disease and tumor size <2 cm, the oncological outcomes of ARH and LRH are similar whether the tumor is visible or not, suggesting that laparoscopic surgery may be suitable for these patients. This study also adds to the evidence that LRH may be suitable for select patients with stage IB1 disease, but more studies are needed to clarify the indications of LRH for cervical cancer.
ACKNOWLEDGEMENTS
We thank Min Hao (The second hospital of ShanXi medical university), Wuliang Wang (The Second Affiliated Hospital of Zhengzhou University), Shan Kang (The Forth Hospital of Hebei Medical University), Bin Ling (China-Japan Friendship Hospital), Lixin Sun and Hongwei Zhao (Shanxi Cancer Hospital), Jihong Liu and Lizhi Liang (Sun Yat-sen University Cancer Center), Lihong Lin and Yu Guo (Anyang Tumor Hospital), Li Wang (The Affiliated Tumor Hospital of Zhengzhou University), Weidong Zhao (Anhui Provincial Cancer Hospital), Wentong Liang (Guizhou Provincial People's Hospital), Shaoguang Wang (The Affiliated Yantai Yuhuangding Hospital of Qingdao University), Xuemei Zhan and Mingwei Li (Jiangmen Central Hospital), Weifeng Zhang (Ningbo Women & Children's Hospital), Peiyan Du (The Affiliated Cancer Hospital and Institute of Guangzhou Medical University), Ziyu Fang (Liuzhou Workers' Hospital), Rui Yang (Shenzhen Hospital of Peking University), Long Chen (Qingdao Municipal Hospital), Encheng Dai and Ruilei Liu (Linyi People's Hospital), Yuanli He and Mubiao Liu (Zhujiang Hospital, Southern Medical University), Jilong Yao and Zhihua Liu (Shenzhen Maternity & Child Health Hospital), Xueqin Wang (The Fifth Affiliated Hospital of Southern Medical University), Ben Ma (Guangzhou First People's Hospital), Zhonghai Wang (Shenzhen Nanshan People's Hospital), Lin Zhu (The Second Hospital of Shandong University), Hongxin Pan (The Third Affiliated Hospital of Shenzhen University), Qianyong Zhu (No.153. Center Hospital of Liberation Army/Hospital No. 988 of the Chinese People's Liberation Army Joint Support Force), Dingyuan Zeng and Zhong Lin (Maternal and Child Health Care Hospital of Liuzhou), Xiaohong Wang (Laiwu People's Hospital/Jinan City People's Hospital) and Bin Zhu (The Affiliated Yiwu Women and Children Hospital of Hangzhou Medical College) for their contribution in data collection.
Footnotes
Funding: This study was initially funded by the National Science and Technology Support Program of China (2014BAI05B03), the National Natural Science Fund of Guangdong (2015A030311024), the Science and the Science and Technology Plan of Guangzhou (158100075), Guangdong Medical Science and Technology Research Fund Project (A2020077), basic and applied basic research fund of Guangdong Province (2019A1515110337) and Nanfang hospital president fund (2019C005).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.P.1, N.Y., L.J.1, L.D., G.J., C.C.
- Data curation: L.P.1, C.L., N.Y., L.J.1, G.J., L.Z.1, J.S., X.Y.
- Formal analysis: L.P.1, L.D., L.Z.2, W.L., B.X.
- Funding acquisition: C.C.
- Methodology: L.P.1, G.J., L.Z.1, J.S., X.Y., L.Z.2, W.L.
- Project administration: L.P.1, C.C., L.J.2, L.P.2
- Resources: N.Y., C.C.
- Software: L.P.1, C.L., L.J.1, L.D., L.Z.2, W.L.
- Supervision: C.C., L.J.2, L.P.2
- Validation: L.P.1, N.Y., L.D.
- Visualization: L.P.1, N.Y.
- Writing - original draft: L.P.1, C.L., N.Y.
- Writing - review & editing: L.P.1, C.L., N.Y., L.J.1, L.D., G.J., L.Z.1, J.S., X.Y, L.Z.2, W.L., W.L., L.J.2, L.P.2, C.C.
1L.P., Pengfei Li; 2L.P., Ping Liu.
1L.J., Jiaqi Liu; 2L.J., Jinghe Lang.
1L.Z., Zhihua Liu; 2L.Z., Zhiqiang Li.
SUPPLEMENTARY MATERIALS
The distribution of urban-rural areas, hospital function, region, and city scale in LRH group and ARH group
Characteristics of patients in the visible tumor subgroup
Characteristics of patients in the invisible tumor subgroup
References
- 1.Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. 1992;166:864–865. doi: 10.1016/0002-9378(92)91351-a. [DOI] [PubMed] [Google Scholar]
- 2.Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. doi: 10.1186/s12885-015-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903–911. doi: 10.1093/annonc/mdr360. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Xu H, Li Y, Wang D, Li J, Yuan J, et al. The outcome of laparoscopic radical hysterectomy and lymphadenectomy for cervical cancer: a prospective analysis of 295 patients. Ann Surg Oncol. 2008;15:2847–2855. doi: 10.1245/s10434-008-0063-3. [DOI] [PubMed] [Google Scholar]
- 5.Gallotta V, Conte C, Federico A, Vizzielli G, Gueli Alletti S, Tortorella L, et al. Robotic versus laparoscopic radical hysterectomy in early cervical cancer: a case matched control study. Eur J Surg Oncol. 2018;44:754–759. doi: 10.1016/j.ejso.2018.01.092. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 7.Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doo DW, Kirkland CT, Griswold LH, McGwin G, Huh WK, Leath CA, 3rd, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: results from a single high volume institution. Gynecol Oncol. 2019;153:242–247. doi: 10.1016/j.ygyno.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. 2019;153:3–12. doi: 10.1016/j.ygyno.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:64–84. doi: 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- 11.Uppal S, Gehrig PA, Peng K, Bixel KL, Matsuo K, Vetter MH, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional retrospective review study. J Clin Oncol. 2020;38:1030–1040. doi: 10.1200/JCO.19.03012. [DOI] [PubMed] [Google Scholar]
- 12.Paik ES, Lim MC, Kim MH, Kim YH, Song ES, Seong SJ, et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028) Gynecol Oncol. 2019;154:547–553. doi: 10.1016/j.ygyno.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Ji M, Li P, Liu P, Zou W, Zhao Z, et al. Comparison between robot-assisted radical hysterectomy and abdominal radical hysterectomy for cervical cancer: a multicentre retrospective study. Gynecol Oncol. 2020;157:429–436. doi: 10.1016/j.ygyno.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Wang W, Liu P, Li P, Wang L, Jin S, et al. Survival after abdominal Q-M type B versus C2 radical hysterectomy for early-stage cervical cancer. Cancer Manag Res. 2019;11:10909–10919. doi: 10.2147/CMAR.S220212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Liu P, Yang Y, Wang L, Liu J, Bin X, et al. Hazard ratio analysis of laparoscopic radical hysterectomy for IA1 with LVSI-IIA2 cervical cancer: identifying the possible contraindications of laparoscopic surgery for cervical cancer. Front Oncol. 2020;10:1002. doi: 10.3389/fonc.2020.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedone Anchora L, Turco LC, Bizzarri N, Capozzi VA, Lombisani A, Chiantera V, et al. How to select early-stage cervical cancer patients still suitable for laparoscopic radical hysterectomy: a propensity-matched study. Ann Surg Oncol. 2020;27:1947–1955. doi: 10.1245/s10434-019-08162-5. [DOI] [PubMed] [Google Scholar]
- 17.Pyeon SY, Hur YJ, Lee JM. Rethinking the next step after unexpected results associated with minimally invasive radical hysterectomy for early cervical cancer. J Gynecol Oncol. 2019;30:e43. doi: 10.3802/jgo.2019.30.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The distribution of urban-rural areas, hospital function, region, and city scale in LRH group and ARH group
Characteristics of patients in the visible tumor subgroup
Characteristics of patients in the invisible tumor subgroup