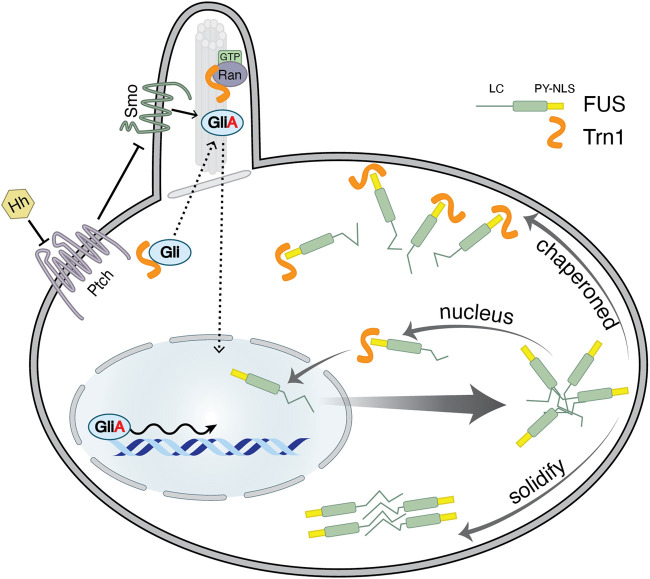
FIGURE 4.
Moonlighting functions of Transportin-1. On one hand, Trn1 has been caught in trafficking proteins destined to cilia (Dishinger et al., 2010; Hurd et al., 2011). For instance, Gli, a transcription factor associated with Hedgehog (Hh) signalling is shown to interact with Trn1 via its ciliary localization signal (CLS) (Han et al., 2017). Ran-GTP in the cilia appears to release Gli from Trn1. Upon Hh signalling Gli is converted to an activated form GliA, a transcriptional activator that then translocates to the nucleus to drive transcription (Kim et al., 2009; Han et al., 2017). On the other hand, Trn1 regulates the aggregation of mislocalized RNA-binding proteins (RBPs) in the cytoplasm (Guo et al., 2018; Hock et al., 2018; Hofweber et al., 2018; Qamar et al., 2018; Yoshizawa et al., 2018). Upon stress or when mutated, FUS translocates into the cytoplasm, where it can aggregate and further solidify into pathologic foci. Trn1 recognizes PY-NLS of FUS and directly drives it back into the nucleus (Dormann et al., 2010). Moreover, Trn1 can additionally bind to the low-complexity (LC) and folded region of FUS. This binding competes with FUS–FUS interaction and protein-assembly. Thus, PY-NLS serves as a cytoplasmic signal such that mislocalized nuclear RBPs are specifically chaperoned by Trn1 (Guo et al., 2018; Hock et al., 2018; Hofweber et al., 2018; Qamar et al., 2018; Yoshizawa et al., 2018).