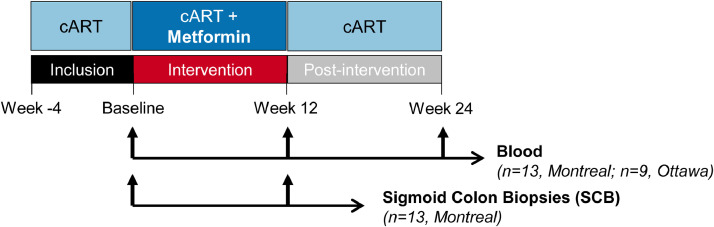
Fig. 1.
Flow chart of the clinical trial. Depicted is the schematic representation of the metformin treatment and the sample collection. Briefly, blood and plasma from HIV-infected individuals with undetectable plasma viral load (<40 HIV-RNA copies/mL) under ART were collected at Baseline, after 12 weeks of metformin treatment (500 to 850 mg b.i.d.) (Week 12) and after 12 week of metformin discontinuation (Week 24) in Montreal (n=13) and in Ottawa (n=9). In the sub-study performed in Montreal, matched sigmoid colon biopsies (SCB) were collected at Baseline and Week 12 (n=13).