Figure 1: The basics of peptidoglycan (PG) synthesis.
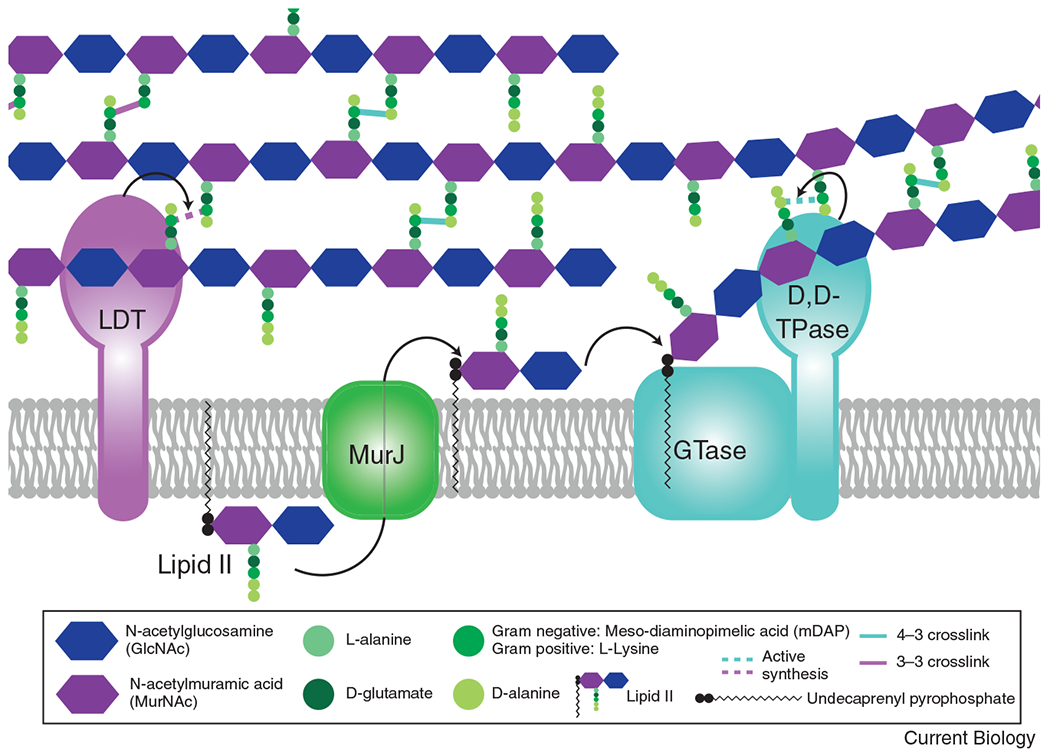
The PG precursor is synthesized in the cytoplasm to create the lipid-linked disaccharide-pentapeptide moiety, lipid II. Lipid II is then flipped across the cytoplasmic membrane by MurJ. Once in the periplasm, a glycosyltransferase (GTase) polymerizes the lipid II moiety onto the growing glycan strand, through the action of either a SEDS protein (RodA or FtsW), a bifunctional class A penicillin binding protein (aPBP), or a GT51 containing enzyme. Once polymerized, the peptide stem can then be crosslinked to a neighboring strand through the action of a transpeptidase (TPase). A monofunctional bPBP (PBP2 or FtsI) or a bifunctional aPBP acts as a D,D-TPase, creating a 4-3 linkage, shown in teal. For simplicity, only the SEDS/bPBP synthases are shown to depict transglycosylation and transpeptidation steps. The peptide stem can alternatively be crosslinked in a 3-3 linkage (shown in purple) through the action of an L,D-transpeptidase (LDT).
