Figure 2: Dynamics and functions of distinct PG synthetic enzymes.
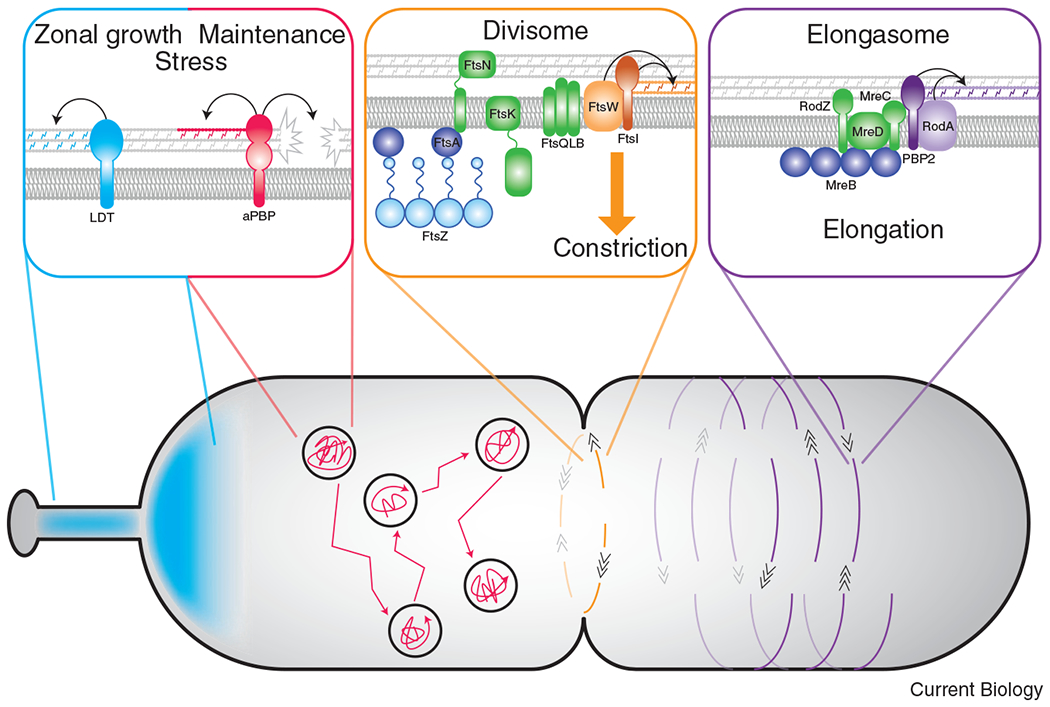
SEDS-bPBP pairs are associated with morphogenetic complexes to direct spatially-regulated PG synthesis, while movement of the aPBPs and LDTs are independent of the division/elongation machinery. Left panel: The importance of LDT activity varies among species. In some organisms, LDT-mediated crosslinking is important in specific regions in a cell, such as during stalk formation or unipolar growth. LDT activity also appears important for resistance to antibiotic treatment. Movement of the aPBPs is diffusive followed by periods of confinement. The bifunctional aPBPs likely act as repair machinery necessary for efficient growth and/or PG synthesis during stress conditions. Middle panel: In almost all bacteria with a cell wall, PG synthesis associated with the divisome is carried out by the FtsW-FtsI enzyme pair. The movement of FtsW-FtsI is bidirectional around the short axis of the division plane, directed by treadmilling of FtsZ filaments. The conserved activation pathway for FtsW-FtsI likely requires the divisome proteins FtsA, FtsK, FtsN, and FtsQLB. Right panel: In many rod-shaped organisms, RodA-PBP2 synthesize PG for elongation and are regulated by elongasome proteins, notably MreC. The elongation machinery moves bidirectionally about the circumference of the short axis of the cell but, unlike the divisome, movement is directed by PG synthesis rather than MreB dynamics.
