Figure 3.
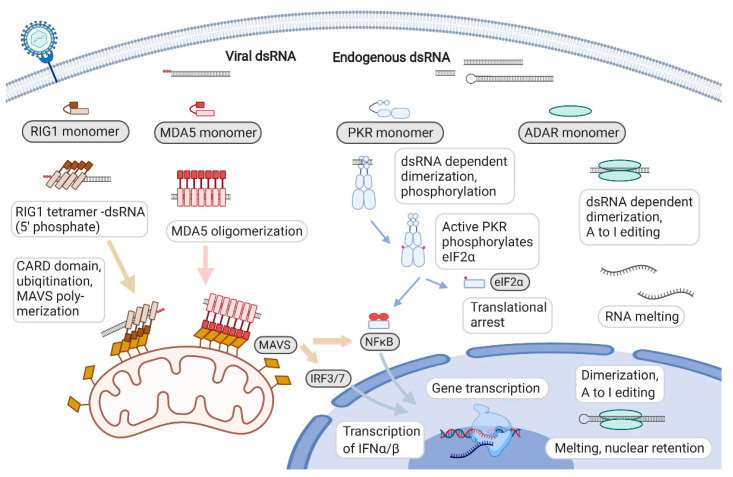
Double-stranded RNA (dsRNA) sensor proteins and activation of innate immunity. Viral dsRNA (including 5′ phosphorylation) or dsRNA from mitochondria and repetitive elements in the cytoplasm are recognized by dsRNA sensors retinoic acid-inducible gene I (RIG1), melanoma differentiation-associated gene 5 (MDA5), protein kinase R (PKR) and ADAR. RIG1 requires the 5′ phosphate group to initiate oligomerization, and MDA5 forms long dsRNA-dependent polymers. Both structures induce mitochondrial antiviral signaling (MAVS) polymerization and, eventually, caspase and interferon signaling. PKR binds short dsRNA molecules, dimerizes and becomes activated through autophosphorylation. Activated PKR dissociates from dsRNA, phosphorylates eukaryotic initiation factor 2α (eIF2α) (which, in turn, inhibits translation globally) and triggers an interferon response. ADAR is present in both the nucleus and cytoplasm and antagonizes dsRNA signaling by melting the RNA hybrid. Figure created with Biorender.com.