Fig. 7.
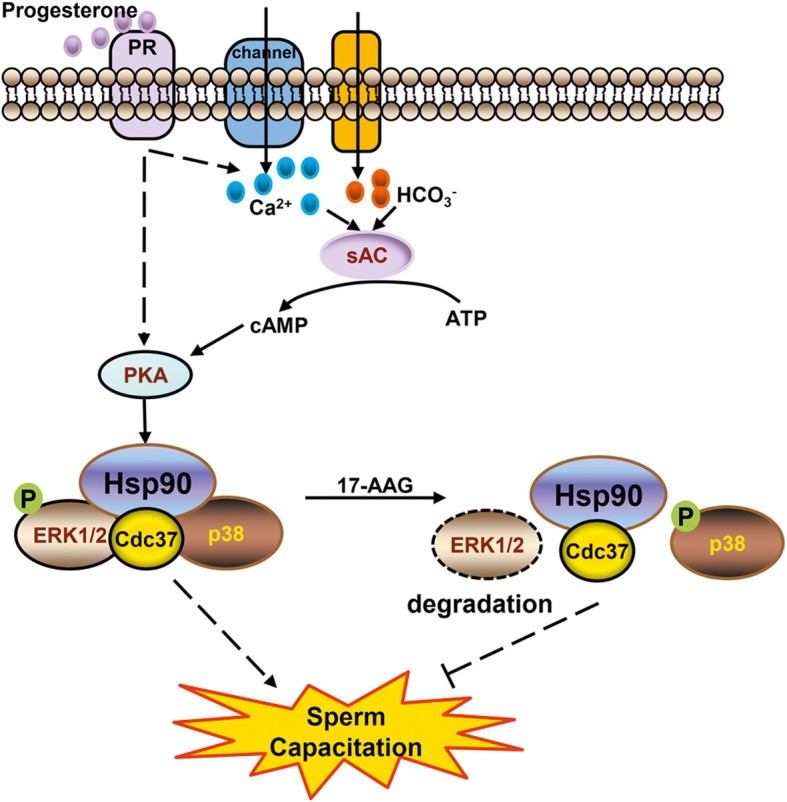
Hsp90 modulates human sperm capacitation via the Erk1/2 and p38 signal pathways
During human sperm capacitation, cholesterol outflow from the plasma membrane increases membrane permeability and thus the influx of Ca2+ and HCO3− activated soluble adenylate cyclase (sAC), which catalyzes the conversion of ATP into cAMP. Subsequently, cAMP activates cAMP-dependent protein kinase A (PKA), which activates target proteins such as Hsp90. Activated Hsp90 and its kinase-specific co-chaperone Cdc37 form a protein complex with Erk1/2, which stabilizes Erk1/2 and maintains its phosphorylation. Phosphorylated Erk1/2 is activated and promotes sperm hyperactivation and the acrosome reaction. Hsp90 and Cdc37 also form a protein complex with p38, which maintains unphosphorylated p38. Since phosphorylated p38 is activated and inhibits sperm hyperactivation, the Hsp90-Cdc37-Erk1/2-p38 complex promotes human sperm capacitation. Treatment with 17-AAG, an Hsp90 specific inhibitor, causes Erk1/2 and p38 to dissociate from the complex. Erk1/2 is subsequently degraded and dephosphorylated, whereas p38 activates itself via autophosphorylation. Ultimately, these changes inhibit human sperm capacitation
