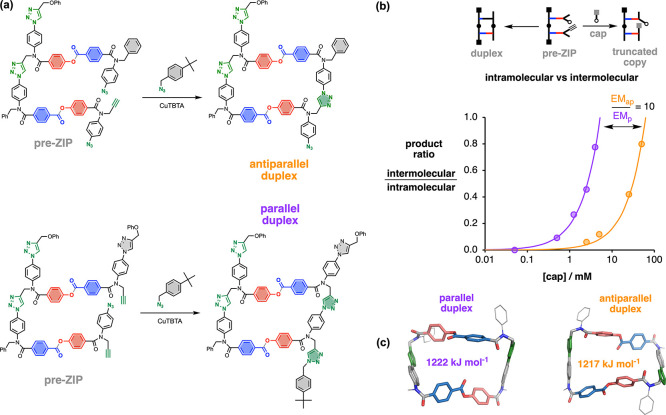
Figure 6.
Precapped monomers determine the backbone direction of the product duplex. (a) The only possible product of the CuAAC reaction is (a) the antiparallel duplex when a terminal alkyne group is removed in the pre-ZIP intermediate or the parallel duplex when a terminal azide group is capped in the pre-ZIP intermediate. (b) Addition of increasing amounts of an external capping agent (4-tert-butylbenzyl azide) was used to determine values of EM through competition with the intramolecular reaction. Ten times more of the capping agent was required to compete with formation of the antiparallel duplex (orange data) than the parallel duplex (purple data). The lines are the theoretical relationships obtained if the product ratio is directly proportional to the ratio of the concentration of the capping agent and the effective molarity for the intramolecular reaction. (c) Molecular mechanics models of isomeric parallel and antiparallel duplexes suggest that the antiparallel backbone arrangement is lower in energy (MMFFs force field with chloroform solvation).2