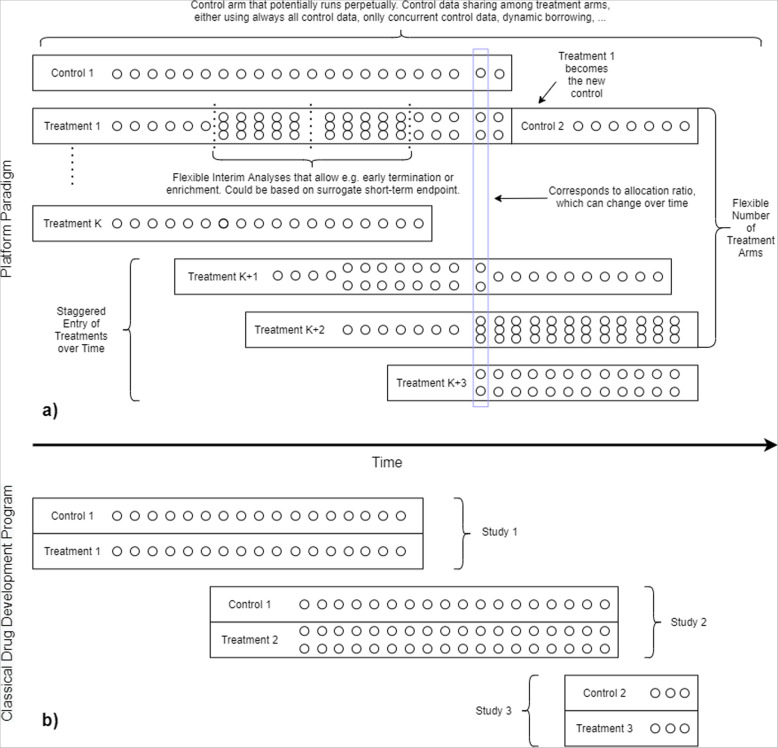
Fig. 1.
Comparison of platform paradigm (a) and classical drug development program (b). Many features can be included in the platform paradigm which are usually not found in classical drug development programs consisting of sequential/parallel two-arm studies, such as a flexible number of interim analyses (possibly using short-term surrogate endpoints), adaptive randomization ratios, a staggered entry of (a flexible number of) treatments over time, change of control treatment within the trial, and different control arm data sharing options