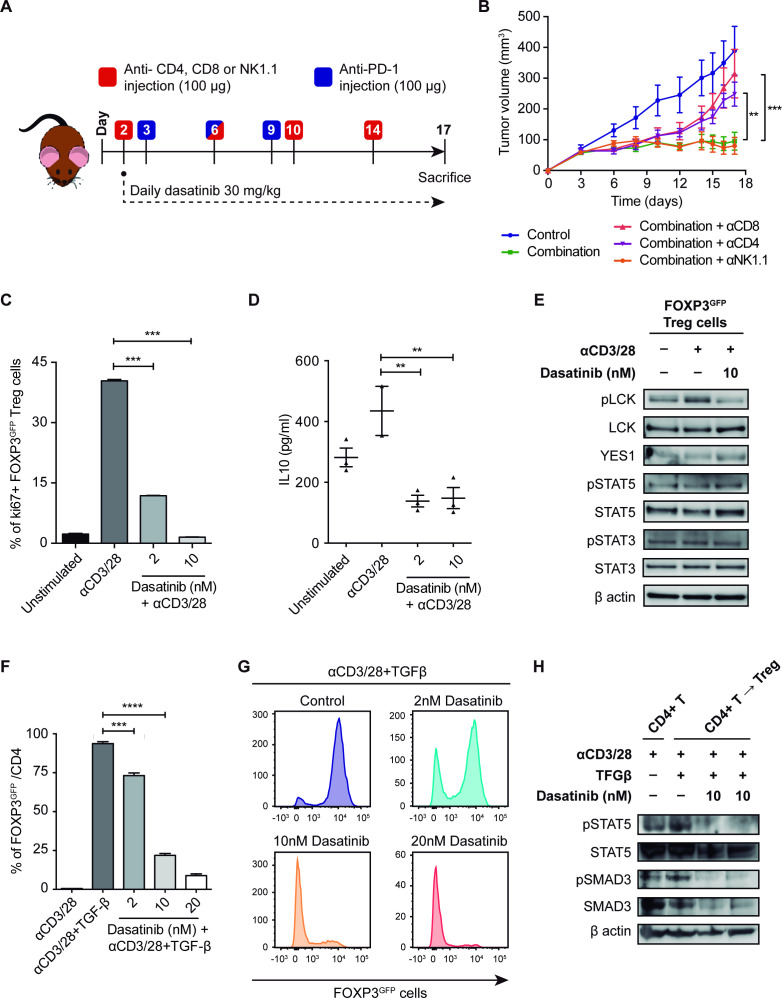
Figure 6.
(A) Schematic representation of the experimental design followed for the depletion of CD8, CD4 and NK1.1 cell populations and the treatment with dasatinib+anti-PD-1 in vivo. (B) Tumor growth of 393P-inoculated cells in the presence of depleting antibodies and treatment with dasatinib+anti-PD-1 (n=6). (C) Treg cell proliferation 4 days after plating in the presence of IL-2, anti(α)CD3, αCD28 and dasatinib (2 and 10 nM) measured by flow cytometry. (D) IL-10 levels measured in Treg cells culture medium after 48 hours of exposure to dasatinib. (E) Protein expression of pLCK, LCK, YES1, pSTAT3, STAT3, pSTAT5 and STAT5 in Treg cells treated with dasatinib (10 nM) for 45 min. (F, G) In vitro experiment of TGF-β-dependent CD4+ T cell conversion into Tregs, in the presence of IL-2, αCD3, αCD28 and dasatinib (2, 10 and 20 nM), analyzed by flow cytometry. (H) Western blotting of pSTAT5, STAT5, pSMAD3 and SMAD3 in CD4+ T cells converted into Tregs after treatment with dasatinib (10 nM). Differences between groups were evaluated with a two-way (B) or a one-way analysis of variance test (C, D, F) followed by a post hoc Bonferroni test. **P<0.01, ***P<0.001, ****P<0.0001. IL, interleukin; LCK, lymphocyte-specific protein tyrosine kinase; PD-1, programmed cell death 1; pLCK, phospholymphocyte-specific protein tyrosine kinase; TGF-β, transforming growth factor beta; Treg, regulatory T cell.