Abstract
Adaptive radiation plays a fundamental role in our understanding of the evolutionary process. However, the concept has provoked strong and differing opinions concerning its definition and nature among researchers studying a wide diversity of systems. Here, we take a broad view of what constitutes an adaptive radiation, and seek to find commonalities among disparate examples, ranging from plants to invertebrate and vertebrate animals, and remote islands to lakes and continents, to better understand processes shared across adaptive radiations. We surveyed many groups to evaluate factors considered important in a large variety of species radiations. In each of these studies, ecological opportunity of some form is identified as a prerequisite for adaptive radiation. However, evolvability, which can be enhanced by hybridization between distantly related species, may play a role in seeding entire radiations. Within radiations, the processes that lead to speciation depend largely on (1) whether the primary drivers of ecological shifts are (a) external to the membership of the radiation itself (mostly divergent or disruptive ecological selection) or (b) due to competition within the radiation membership (interactions among members) subsequent to reproductive isolation in similar environments, and (2) the extent and timing of admixture. These differences translate into different patterns of species accumulation and subsequent patterns of diversity across an adaptive radiation. Adaptive radiations occur in an extraordinary diversity of different ways, and continue to provide rich data for a better understanding of the diversification of life.
Background
Adaptive radiation has been considered the connector that unites ecology and evolution (Givnish and Sytsma 1997). Since capturing the attention of evolutionary biologists when Darwin, using the Galapagos finches, developed his “principle of divergence,” studies of adaptive radiation have been central in developing our understanding of the mechanisms that drive speciation, diversification, and many associated ecological and evolutionary processes (Simpson 1953; Givnish and Sytsma 1997; Schluter 2000; Grant and Grant 2014). However, research on adaptive radiations is often as disparate as the ecologically differentiated species contained within them, which makes generalization of process and patterns across systems difficult. One of the few uniting commonalities is that adaptive radiations generally, though not always (Losos 2010), require ecological opportunity and are associated at some stage with divergent natural selection shaping adaptation to the biotic or abiotic environment (Schluter 2000; Stroud and Losos 2016). Beyond this point, there has been limited consensus on what processes shape adaptive radiations across space, time, and taxa. The current paper arose from a meeting of the American Genetic Association held in Waimea, Hawaii, in July 2018 with the goal of synthesizing our knowledge of ecologically, geographically, and taxonomically diverse radiations (Figure 1) to provide a more general understanding of the diversity of processes that are included under the umbrella of adaptive radiation. We attempt to identify common denominators, where they exist, and to highlight differences, where we think they are real and important, that underlie adaptive radiations, to reinvigorate the search for general framework for explaining when—and how—they occur.
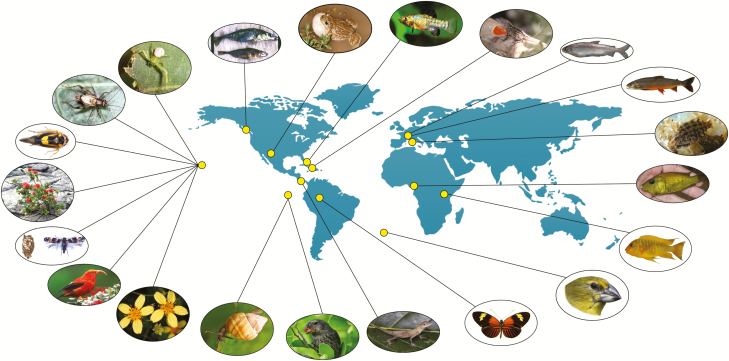
Figure 1.
Model systems studied by contributors of the AGA 2018 President’s Symposium: Origins of Adaptive Radiation. Yellow dots represent areas where field studies have been conducted and do not accurately represent the full geographic distribution of each group. Anti-clockwise from top-right: Mediterranean labrine wrasses, Alpine charr (Salvelinus umbla complex), European Alpine whitefish (Coregonus spp.), Caribbean Anolis lizards, San Salvador pupfish (Cyprinodon sp.), spadefoot toads (Spea sp.), stickleback fish (Gasterosteus aculeatus), Hawaiian spiders, Laupala crickets, Nesophrosyne leafhoppers, Hawaiian Metrosideros plants, Hyposmocoma moths, Hawaiian honeycreepers, Hawaiian Bidens, Galapagos land snails (Bulimulus sp.), Darwin’s finches (Geospiza sp.), mainland Anolis lizards, Heliconius butterflies, Nesospiza finches of the Tristan da Cunha archipelago, African Great Lake cichlids, and Cameroon crater lake cichlids. Photography credits anti-clockwise from top right: O. Seehausen, O. Seehausen, O. Seehausen, J. Stroud, C. Martin, D. Pfennig, A. Hendry, R. Gillespie, K. Shaw, G. Bennett, E. Stacy, D. Rubinoff, J. Jeffreys, M. Knope, C. Parent, A. Hendry, J. Stroud, J. Mallet, P. Ryan, C. Wagner, C. Martin.
What Do We Mean by “Adaptive Radiation?”
The definition of adaptive radiation has been elusive, as the term has been used for a broad array of situations from the classically recognized rapid adaptive radiations of Galapagos finches and African Great lakes cichlids, to the striking, but slow, radiations of Greater Antilles Anolis lizards, and Brocchinia bromeliads in the South American tepuis (Givnish 2015), and from intraspecific divergence (Hendry et al. 2009) to ancient divergences among major lineages (Burns and Sidlauskas 2019). The term has also been used to describe species that are largely allopatric (Murray et al. 1993) and single species showing divergent feeding behavior (Knudsen et al. 2010), to much more diverse clades of insects (Bennett and O’Grady 2013; O’Grady and DeSalle 2018), and spiders that co-occur syntopically within a given island (Gillespie 2016), as well as everything in between. Furthermore, debate over the distinction between adaptive and nonadaptive radiations continues (Czekanski-Moir and Rundell 2019), in particular, because (1) nonadaptive radiation (the formation of multiple species that are ecologically similar) can sometimes give way to classic adaptive radiation as newly formed species develop ecological differences in the course of diversification (Rundell and Price 2009); such ecological divergence can be tied to interactions with ecologically similar close relatives (see below). Alternatively, (2) ecological separation may be largely limited to divergences at the onset of the radiation, with subsequent speciation events over the course of the radiation occurring in isolation without major ecological shifts. Clearly, different processes are involved in adaptive radiation, adding to confusion in its use (Olson and Arroyo-Santos 2009).
Attempting to resolve problems inherent in the term, a number of authors have proposed new and improved definitions of adaptive radiation, as well as criteria for demonstrating when one has or has not occurred. Perhaps the most widely accepted definition currently is that proposed by Schluter (2000)—the evolution of ecological diversity within a rapidly multiplying lineage; this is evaluated by a set of four criteria, (1) common ancestry, (2) phenotype-environment correlation, (3) trait utility, and (4) rapid speciation. It has proven exceptionally difficult, however, for most studies to satisfy all these criteria (Rundell and Price 2009). As a result, the number of cases that can be considered “adaptive radiations” under these criteria is relatively few. At the other extreme are definitions that are broadly inclusive. Such definitions include the ‘evolutionary divergence of members of a single monophyletic lineage into a variety of adaptive forms’ (Futuyma 1998; Losos 2010); a ‘pattern of species diversification in which different species within a lineage occupy a diversity of ecological roles, with associated adaptations’ (Gillespie et al. 2001); and the ‘rise of a diversity of ecological roles and associated adaptations within a lineage, accompanied by an unusually high level or rate of accumulation of morphological/physiological/behavioral disparity and ecological divergence’ (Givnish 2015). As an alternative to emphasizing a definition, other authors have sought to separate the different components of the phenomenon—treating rate separately from ecological and morphological disparity (Donoghue and Sanderson 2015; Salzburger 2018), or by dividing the phenomenon into various components, such as 1) multiplication of species of common descent, 2) adaptation via natural selection, and 3) extraordinary diversification; testing for each criterion separately (Glor 2010). Importantly, it is clear that adaptive radiation covers many different situations. Hence, treating it as a single phenomenon can preclude understanding of the interplay between factors including isolation, selection from the external environment, and interactions between close relatives within the radiation, in generating diversity within a given radiation, and how these differences may affect patterns of species accumulation through time.
Our goal in the current paper is not to defend a specific definition or concept but rather to embrace the diversity of viewpoints on the topic. Our overarching message is that progress in the field requires clear identification of the nature and timing of both speciation and ecological diversification. We begin our examination of adaptive radiation by outlining three elements that are necessary, though not fully sufficient to explain adaptive radiation—ecological opportunity, time, and adaptive response to ecological selection (Schluter 2000).
Attributes Common to Adaptive Radiation—Opportunity, Time, and Adaptive Response
Opportunity and Ecological Arena
Understanding adaptive radiation requires a joint focus on both ecological and evolutionary processes, and how each influences the other. Simpson (1953) proposed that the primary prerequisite for adaptive radiation to occur is ecological opportunity, which can arise in one of three ways: 1) colonization of underpopulated or underutilized areas; 2) a key innovation that allows a lineage to interact with the environment in a novel way; or 3) extinction of a previously dominant group. The radiations examined here are all extant and largely without good fossil records; we do not consider radiations that have been largely eliminated through extinction (Morlon et al. 2011) nor do we address the importance of extinction in facilitating subsequent radiation (Feduccia 2003; Chen and Benton 2012; O’Leary et al. 2013; Hull 2015). As initially hypothesized (Simpson 1944, 1953), ecological opportunity arises in the form of ecological space that is unoccupied or underutilized by competing taxa, and that permits evolutionary diversification (Schluter 2000; Losos 2010). However, the opportunity provided by open ecological space is relative to the taxon in question and the response of a taxon to opportunity is necessarily dictated by niche discordance in concert with niche availability (Wellborn and Langerhans 2015).
A powerful form of ecological opportunity that affects many lineages is the colonization of novel habitats or areas that lack ecologically similar species largely due to barriers that limit colonization, such as geographic isolation. Situations providing ecological opportunity are perhaps most frequent on remote or newly formed islands and lakes, or upon adoption of a novel host or pollinator (Ehrlich and Raven 1964; Wheat et al. 2007). The ecological opportunity thus provided is an attribute of the community, rather than a given lineage although clearly the taxon must have attributes that allow it to take advantage of the ecological opportunity, such as ecological versatility (Stroud and Losos 2016). As such, ecological opportunity is related to the “taxon cycle” hypothesis (Wilson 1961; Ricklefs and Bermingham 2002a), in which early colonists to a site are successful and abundant, potentially due to “enemy release” and subsequently diversify into different specialized ecological niches. Thus, the response to ecological opportunity is linked to a shift in the balance between competitors, predators and prey, and/or parasites and hosts (Warren et al. 2015).
The importance of ecological opportunity points to the order of colonization as a key factor in dictating which lineages radiate and which do not. Priority effects from a diversifying lineage may prevent subsequent lineages from gaining a foothold or subsequently diversifying (Fukami et al. 2007; Fukami 2015; De Meester et al. 2016). Alternatively, a taxon in a nonradiating lineage may establish first, and remain limited to the ancestral niche; the presence of this lineage could preclude establishment by a secondary colonizer from the same lineage within that ancestral niche space, potentially facilitating ecological exploration in the secondary colonizer.
The amount of genetic variation will dictate the ability of a colonizing population in the new space to respond to selection and the rate of adaptive divergence from its mainland ancestor, whereas the degree of partial or complete geographic isolation combined with the dispersal capacity of taxa in the regional species pool will influence the rate at which the habitat can be filled with other colonizing species, reducing ecological opportunity for adaptive radiation. Subsequent diversification within the lineage will be shaped by the interplay of geographic separation, resources, competitors, predators, and parasites that will all change through time. Time is thus crucial in the “race” between adaptation and immigration (Emerson and Gillespie 2008; Gillespie and Baldwin 2010; Vanoverbeke et al. 2016). In the Hawaiian Islands, for example, the oldest of the current high islands (Kauai, ca. 5 million years old) emerged at a time when the previous islands were low and far apart (Price and Clague 2002). With the profound isolation from other high islands for ca. 1 Ma, there was greater time and opportunity for ecological exploration and diversification on Kauai (Gillespie 2016). Subsequent appearance of younger islands has been associated with increased opportunity for island hopping (Lerner et al. 2011) and hence less time for ecological exploration by a single lineage within an island. As a result, a number of lineages are characterized by ecological diversification on the oldest islands only, with colonization of the younger islands by island hopping of previously diverged ecological forms, as has been shown, for example, in flies (Magnacca and Price 2015), leafhoppers (Bennett and O’Grady 2013), and spiders (Garb and Gillespie 2009). Within any given radiation, the tendency for lineages to progress from older to younger islands (referred to as the ‘progression rule’) appears to be indicative of strong priority effects associated with original establishment on older islands inhibiting back colonization from younger islands (Shaw and Gillespie 2016).
Time and Rate
Adaptive radiation is frequently associated with an increase in the rate, or “early bursts” of species diversification as ecological opportunity is explored, followed by a slow down as niche space fills up, as has been shown in some classic adaptive radiations (Gavrilets and Losos 2009; Rabosky et al. 2013). However, adaptive shifts can occur without increased rates of diversification, as demonstrated in lineages of bromeliads in South America (Givnish 2015), assassin spiders (Wood et al. 2013), and vanga birds (Reddy et al. 2012) in Madagascar. And, finally, adaptive radiations are often associated with increased rates without any evidence for a slow down (Harmon et al. 2010); situations where diversification is adaptive without any increase in the rate of speciation could arise, for example, if the ancestral taxon has low levels of standing genetic or trait variation to allow adaptation to novel habitats. In this case, founding populations must rely on new mutations to catalyze each successive adaptive shift.
Adaptive Response
Radiations have been broadly characterized as adaptive or nonadaptive (e.g., Rundell and Price 2009), depending on the extent to which species have diversified ecologically. While classic adaptive radiation involves ecological shifts, nonadaptive radiations (clades that exhibit little ecological disparity) show ecological conservatism—at least in traits that can be easily measured—over evolutionary time scales. Initially defined as ‘‘evolutionary diversification from a single ancestor, not accompanied by relevant niche differentiation’’ (Gittenberger 1991), nonadaptive radiations are common in taxa with low dispersal ability, as in many (not all) snail and salamander lineages, that are hence easily isolated when their habitats become spatially subdivided (Wake 2006). Species formation in large radiations, however, can involve complex mixtures of niche divergence and niche conservatism (see below).
In summary, ecological opportunity, time, and adaptive response are necessary, although not fully sufficient, ingredients of all adaptive radiations surveyed here. The role that each of these factors plays, however, can vary considerably across radiations and even over time within a radiation. Clearly needed are analyses across multiple radiations that can examine how and when species diverge during the course of adaptive radiation. Given the variety of mechanisms through which adaptive radiation may be achieved, we compared a diversity of adaptive radiations studied by the authors to tap the experience and knowledge they have garnered of their respective research systems. Our hope is to discern common denominators and characterize differences in ways that can help guide further investigation.
Common Denominators Across Adaptive Radiations—Questions and Answers
After the conference, contributors were asked to address seven questions in relation to their study systems, with predefined alternatives from which to choose, and given freedom to speculate. The lineages under consideration, and on which the authors are experts, included: Hawaiian Bidens and Metrosideros plants; Galapagos Naesiotus land snails; Hawaiian Tetragnatha, Ariamnes, and Mecaphesa spiders; Hawaiian Laupala crickets; Hawaiian Nesophrosyne leafhoppers; Hawaiian Drosophila flies; Hawaiian Hyposmocoma moths; South American Heliconius butterflies; Rhagoletis fruit flies; North American threespine stickleback fish; East African cichlid fishes; pre-Alpine European whitefish; Mediterranean labrine wrasses; San Salvador Island pupfishes; Cameroon crater lake cichlid fish; Eastern plethodontid salamanders (glutinosus group); Anolis lizards of the Greater Antilles; mainland Anolis (subclade Draconura); Darwin’s finches; Tristan finches; and Hawaiian honeycreepers. These study systems are, of course, a partial and perhaps biased representation of all adaptive radiations. Nonetheless, they cover a diversity of taxonomic groups and geographic settings from which we seek to identify commonalities. The results suggest general principles that might be explored in other systems.
The answers (26–28 responses for each question) are given in Supplemental Figure 1 and summarized below:
How did your lineage gain access to the (novel/underutilized) eco-evolutionary space into which it radiated? The question here related to the role of ecological opportunity associated with geographic colonization of a new environment, or key innovations coupled with colonization of a new set of niches. For the radiations examined, geographic colonization was the most common factor identified (78%), sometimes in conjunction with a key innovation (18%).
How does the ancestral niche compare to what you know of the pattern of establishment of niches between species within the radiation? It is often difficult to determine whether the ancestor of a radiation was a generalist, or whether the generalist strategy arose during ecological release upon colonization of new ecological space. However, for the majority of lineages, including cichlids, Anolis lizards, Hawaiian insects, and Metrosideros plants, contributors speculated that the ancestral species was most often a generalist (43%), with subsequent diversification leading to multiple specialist species. In Hawaiian insects that feed on plants, colonizing ancestors may frequently have arisen from generalists that might have been polyphagous in their ancestral range, facilitating establishment in an ecosystem with restricted and depauperate flora (Bennett and O’Grady 2012). However, in other lineages (25%), it appears that the ancestor was likely specialized and underwent ecological release upon colonization of the islands, most notably for Hawaiian spiders, moths and crickets (Otte 1994), Galapagos snails, and possibly stickleback fish. In Hawaiian Tetragnatha spiders, for example, the sister lineage on the American mainland is widespread but restricted to riparian habitats, building flimsy webs over water, whereas the species radiation in Hawaii is found in almost every forest habitat and microhabitat (Gillespie 2016). Likewise, the most probable sister group to Galapagos Naesiotus snails is restricted to dry forest habitats, whereas Galapagos snails have adapted to a much wider range of habitats (C. Parent, unpubl) (Phillips et al. 2020). Similarly, phylogenetic reconstruction of extant Hawaiian honeycreepers suggests that the Cardueline colonizer was a finch-billed, seed-eating specialist; this morphology seems to have been lost at the onset of the honeycreeper radiation (Campana et al. 2020), with the finch morphology subsequently regained from a thin-billed ancestor (Lerner et al. 2011).
Whether ancestors were generalist or specialist, most radiations are associated with expansion of total niche breadth beyond that of the ancestral range, as has been shown in cichlids (Joyce et al. 2005) and in Hawaiian insects (Bennett and O’Grady 2012; Bennett and O’Grady 2013), likely due to both release from competition and/or release from predation and parasitism. A generalist ancestor can give rise to multiple descendant species that are not simply partitioning broad niche space, but are also (often greatly) expanding total niche breadth across the descendant species that exceeds that of the generalist common ancestor (e.g., Rubinoff and Schmitz 2010). In Hawaiian Metrosideros, population genetic analyses suggest the evolution of habitat specialists from a widespread more generalist taxon but with overall increase in niche breadth across the different species of the radiation (Stacy et al. 2014, 2020; Stacy and Sakishima in review). Members within a radiation are variably specialized, with some members no more specialized than the ancestor and some perhaps even less, with a classic example from Galapagos finches; that is, while the ancestral colonizer is not certain, the oldest species in the radiation are very specialized and some of the younger species in the radiation are broad generalists (Grant 1999; De León et al. 2014).
Tephritid fruit flies in the Rhagoletis pomonella sibling species group highlight an additional important consideration of standing ecological variation (or environmental plasticity) in regard to the question of specialist versus generalist. Rhagoletis flies attack the fruit of different host plants and adaptively radiated via a series of sympatric host shifts from an ancestral hawthorn-infesting population (Bush 1969; Berlocher and Feder 2002). Thus, while the ancestor may be a specialist, a key trait involved in host shifting is the timing and synchronization of pupal diapause with host availability (Dambroski and Feder 2007). As a result, taxa are allochronically reproductively isolated. The ancestral hawthorn-infesting taxon, while a hawthorn specialist, shows latitudinal genetic variation in eclosion timing according to hawthorn fruiting schedules (Doellman et al. 2019), providing polymorphism to enable local shifts and ecological specialization on new hosts with varying fruiting times. Thus, diversification occurs in communities that are already rather full (Cornell 2013), as also may be the case in the Heliconius butterflies (Merrill et al. 2015).
III. In the initial establishment of the radiation, what is the pattern of niche occupation? This question addressed whether the radiation started by (1) initial establishment in a preferred niche and exclusion or nonappearance of subsequent colonists, followed by radiation into many other niches (Leigh et al. 2007), or alternatively, (2) exclusion from the ancestral niche (perhaps by earlier colonists which did not radiate) leading to initial establishment in novel niches and associated radiation. Many contributors (39%) considered that initial establishment occurred in the preferred niche with subsequent colonists excluded (cf. priority effects; e.g. in Laupala crickets Shaw and Gillespie 2016). Variations on these ideas were suggested for cichlids and Mediterranean labrine wrasses with initial establishment in the niche resembling the ancestral niche, although subsequent colonists were not excluded from that same niche even though they had substantial niche overlap (both in microhabitat and trophic resources) with the earlier colonists. Here, ensuing radiation has occurred by rapid “cladistic expansion” from this niche into many other niches. However, opinions varied widely even for the same lineage, likely reflecting the difficulties in obtaining data that would support one or the other scenario. Indeed, without a timeline, distinguishing between initial colonization in an ancestral niche with a subsequent shift versus direct colonization in a new niche without that first step into the ancestral niche, is challenging.
IV. In the course of adaptive radiation, what factors drive divergence between populations, some of which become species? There are two clear mechanisms through which initial reproductive isolation can occur. The first is ecological—divergent selection between different environmental conditions; the second is divergence in isolation without divergent ecological selection, though there may be sexually mediated divergent selection, and ecological divergence may arise subsequently due to biotic interactions.
Divergent or disruptive selection between different environmental conditions—This mechanism was suggested for all plants, fishes, Galapagos and Tristan finches, and Rhagoletis flies (46% responses). The numerous forms of Metrosideros apparently formed and persist by divergent selection with genetic incompatibilities contributing to partial reproductive isolation in hybrid zones (Stacy et al. 2017), with differential adaptation across successional (Morrison and Stacy 2014), elevational (Stacy et al. 2020), and riparian (Ekar et al. 2019 ) gradients. In Tristan finches (Nesospiza spp.), the original colonizers were small-billed (Stervander 2015); the arrival of a novel food source (fruits of the island tree Phylica arborea) introduced disruptive selection pressure, which resulted in a miniature radiation into replicate taxon pairs of small- and large-billed finches on each of two islands (Ryan et al. 2007; Stervander 2015). Similarly, selection for different environments, such as that associated with color pattern mimicry or host choice, appears to be the initial driver of divergence in Heliconius butterflies, and this drives changes leading to assortative mating based on color pattern and microhabitat (Merrill et al. 2015). Although genetic incompatibilities arise and are important even within some species, the strongest initial barriers between species appear to be predominantly ecological and sexual.
The distinction between divergent and disruptive selection is that the former occurs between populations and the latter occurs within them. Both contexts are found in adaptive radiations. In Lake Victoria cichlids, initial ecological selection between niches is often divergent rather than disruptive; however, disruptive selection emerges from the interaction of sexual selection with the environment (Seehausen et al. 2008; Moser et al. 2018; van Rijssel et al. 2018). Disruptive selection is evident in other cases of Lake Victoria cichlids, albeit few, such as in the genus Neochromis (van Rijssel et al. 2018), in Cameroon crater lake cichlids (Martin 2012), Tristan finches (Ryan et al. 2007), and Darwin’s finches (Hendry et al. 2008). In some stickleback and Cameroon cichlids, disruptive selection gradients were only moderate in strength, suggesting that ecological selection was not sufficient to drive species divergence (e.g., Matessi et al. 2002; Bürger et al. 2006; Bolnick 2011) Alternatively, in one Cameroon cichlid radiation it appears that an influx of additional genetic variation for olfactory signals was the primary driver of speciation (Poelstra et al. 2018). In the pre-Alpine whitefish radiation (Hudson et al. 2011; Vonlanthen et al. 2012), divergent selection occurs between different spawning habitats (water depth), possibly coupled with disruptive selection on trophic adaptations in the feeding habitat (which is distinct from spawning habitat in these radiations).
Host shifts are linked to speciation events among native Hawaiian leafhoppers (Hemiptera: Nesophrosyne), though much of the ecological diversity among the >200 species in this lineage has resulted from ecological divergence between host-plants at the onset of the radiation (Bennett and O’Grady 2012) with subsequent diversification in allopatry without host shifts between islands (Bennett and O’Grady 2013). Symbiotic interactions with microbes may provide another—although currently poorly understood—evolutionary mechanism that may facilitate adaptive shifts and adaptive radiation more broadly (Poff et al. 2017). Symbionts are known to provide a number of beneficial traits to their hosts, permitting them to use resources and to persist in environments that may otherwise be unsuitable for hosts (Bennett and Moran 2015).
Divergence in isolation without (initial) ecological selection—A second mechanism through which initial divergence can occur is through intrinsic reproductive incompatibility that is ecologically independent (32% responses, or 46% in conjunction with divergent selection). Thus, anoles (Losos 2009; Stroud and Losos 2020), Hawaiian spiders (Cotoras et al. 2018; Gillespie 2005), and Galapagos snails (Phillips et al. 2020), all appear to demonstrate initial divergence in the same environment, though in allopatry presumably through intrinsic incompatibility. Ecological shifts are associated with subsequent secondary contact (Cotoras et al. 2018; Stroud and Losos 2020).
In a few situations (4% responses), contributors chose neither of the above responses for their lineage; rather, they suggested that divergence in isolation may be a slow process and lead to nonadaptive radiation. Thus, in the plethodontid salamanders of the eastern United States, populations became isolated following the formation of the Appalachian mountain range (Kozak et al. 2006). Isolated populations were subsequently unable to maintain connectivity and diversified into ecologically similar and morphologically cryptic allo- or parapatric species that replace each other geographically (Kozak and Wiens 2010). Among spiders in the Hawaiian Islands, nonadaptive radiation has been well described in Orsonwelles (Linyphiidae) with 13 species across the islands: all species have similar ecologies, and species tend not to co-occur (Hormiga et al. 2003). Similar patterns of allo- and parapatric replacement of members within a lineage have been documented in many lineages including Galapagos mockingbirds (Arbogast et al. 2006), Galapagos tortoises (Beheregaray et al. 2004), Lake Malawi (Allender et al. 2003), and Lake Victoria cichlid fish (Seehausen et al. 1999), although there is often some difference between the environments occupied by the different taxa.
Sexual selection can also play a role in initial reproductive isolation without major ecological shifts and lead to very rapid diversification (4% responses). Thus, members of the native Hawaiian crickets in the genus Laupala share a similar niche but still display species coexistence with up to 4 species in sympatry. Although the specific mechanism of sexual selection is unknown, selection likely plays a role in speciation in this group producing sexually rather than ecologically differentiated groups (Otte 1994; Mendelson and Shaw 2005; Xu and Shaw 2019). However, since divergent sexual selection is often tied to ecology (e.g., Maan and Seehausen 2011), the distinction between adaptive and nonadaptive radiation can become blurred.
V. In the course of adaptive radiation, do species have long-term persistence or are they ephemeral? This question asks whether most entities persist, once formed; or whether they are ephemeral, eliminated by ecological or evolutionary processes of exclusion, introgression upon secondary contact, reversal of speciation, or demographic stochasticity (Rosenblum et al. 2012; Seehausen et al. 2008). These ideas build on those of ephemeral diversification, wherein most diverging groups never diverge to the point of being permanently isolated species (Futuyma 1987). The opinions of contributors were divided between those that considered the focal lineages were ephemeral (39%) versus persistent (39%). The fate of ephemeral forms varied among lineages. In sticklebacks, it appears likely that many freshwater forms are ephemeral and have been extirpated by multiple mechanisms, including demographic stochasticity in addition to environmental processes and introgression. For instance, ice ages likely obliterated most freshwater forms of stickleback, such that many of today’s forms have evolved from marine forms only since the most recent glaciation. Likewise in pupfishes, reproductively isolated ecotypes may routinely go extinct due to environmental or geological processes such as loss of hypersaline lake environments. For radiations involving slow-to-speciate taxa such as Hawaiian Metrosideros trees, the presence of multiple morphologically distinct yet weakly genetically diverged forms may result from the lack of persistent divergent selection on unstable volcanic islands (Stacy et al. 2020) and species boundaries will likely disappear through introgression in this highly interfertile group. Likewise in Hawaiian Bidens, species are generally fully isolated either by geography (on different islands) and/or by habitat (and pollination syndrome for the one bird pollinated B. cosmoides on Kauai), but when secondary contact occurs the species can meld back together into hybrid swarms since intrinsic reproductive isolation has not yet occurred amongst any of the endemic Hawaiian species tested (Ganders and Nagata 1984; Knope et al. 2013; Knope et al. 2020). However, all Hawaiian species tested are intrinsically isolated, likely by genetic incompatibilities, from taxa in their hypothesized Central American sister clade (Knope et al. 2013), and this reproductive incompatibility appears to have arisen within the past ~2 My (Knope et al. 2012, 2020).
Genetic evidence suggests widespread mtDNA leakage in Hawaiian Laupala crickets, suggesting persistent hybridization across the radiation (Shaw 2002; Shaw and Gillespie 2016); nonetheless, two clades of this group have maintained genetic distinctiveness in sympatry for at least 3.5 My (Mendelson and Shaw 2005). In Heliconius butterflies, where local sympatry of sister species is widespread, gene flow plays a role in persistence of species (Rosser et al. 2015); here, species differentiation is maintained by the occupation of different niches and assortative mating, potentially aided by F1 female hybrid sterility and pleiotropic effects of mimicry, habitat and host plant shift leading to assortative mating (Merrill et al. 2015).
In Lake Victoria cichlids, timelines are very short, yet >500 species have evolved in a time frame similar to that of sticklebacks, the latter having evolved at most two species in sympatry. Thus, most cichlid taxa are predicted to persist at least for thousands of years (which is long in a 15,000-years-young radiation), but some are likely to have been eliminated by speciation reversal. The scale of speciation reversal is mediated by environmental change (natural and anthropogenic), and the impact can be massive in parts of the radiation.
VI. In the course of adaptive radiation, which factors best describe achievement of species co-occurrence? Contributors working on Caribbean Anolis (Losos 2009; Stroud and Losos 2020), Hawaiian spiders (Cotoras et al. 2018), Hawaiian Drosophila fruitflies, Hawaiian Nesophrosyne leafhoppers, and Darwin’s finches (43% responses) argued that new incipient species often share ecological requirements when they come into secondary contact. Here, character displacement—potentially arising from plasticity in ecological traits (Pfennig and Pfennig 2012b)—gives rise to ecological divergence in sympatry (Brown and Wilson 1956). Likewise, among South American mainland radiations of Heliconius butterflies, ecological character displacement may begin very early during divergence to become the major driving force of speciation with gene flow (Rosser et al. 2015). However, in the Hawaiian Laupala crickets, species are largely similar in ecology, with the most closely related species largely allo- or parapatric; divergence in mate recognition apparently stabilizes taxa in sympatry without ecological displacement (Xu and Shaw 2020).
In other groups (21% responses), some form of ecological divergence appears to be involved prior to sympatry of taxa. In sympatric stickleback species pairs (see above), ecological character displacement is facilitated by initial divergence between environments. In Lake Victoria cichlids likewise, co-occurrence appears to often come about perhaps via having somewhat distinct ecologies that evolved during parapatric speciation before coming back into full sympatry (Figure 2, Seehausen 2015); however, character displacement likely also plays a role (van Rijssel et al. 2018). In Hawaiian Metrosideros trees, ecological divergence with gene flow may best explain the origin of morphotypes, given its exceptional dispersibility (Dawson and Stemmermann 1990). Similarly, in Rhagoletis flies, where there is no evidence for character displacement, ecological divergence with gene flow via host shifting is initially responsible for the divergence and co-occurrence of taxa (Bush 1969; Berlocher and Feder 2002).
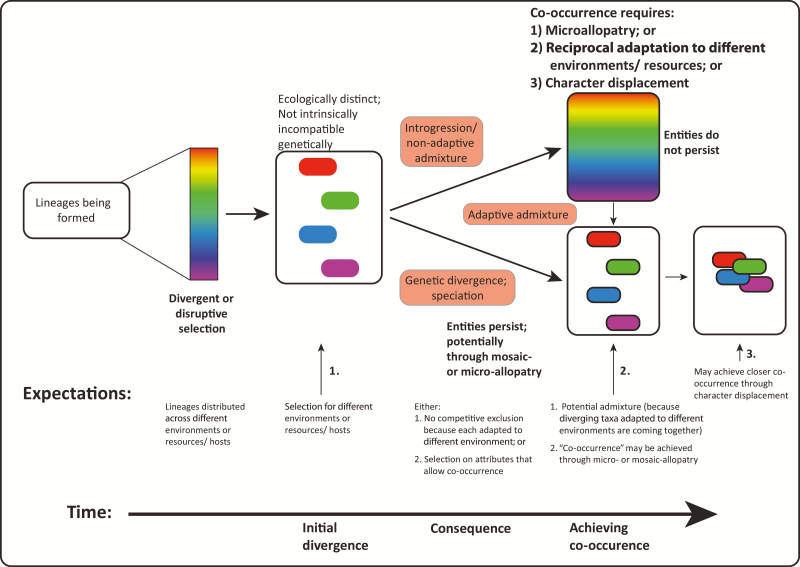
Figure 2.
Contrasting roles of: (1) factors external to the membership of the radiation coupled with divergent or disruptive selection associated with the environmental conditions or resource or host use; versus (2) reproductive incompatibility within the same environment fostering initial divergence, with ecological divergence, if it occurs, happening later and associated with interactions between relatives internal to the radiation. Part (1) is detailed further in Figure 3; part (2) in Figure 4.
VII. What are the underlying genetic and demographic conditions that lead to ecological disparity? The first part of this question (Supplementary Figure S1, VIIa) addressed the relative importance of admixture, developmental plasticity, evolvability (standing variation and the potential for new mutation), and/or lineage priority in paving the way for ecological disparity. Clearly, all of these processes may play a role and it is their interaction that may promote adaptive diversification; essentially all of the contributors at the conference answered the question in this manner. In several groups (25%), hybridization likely contributes to diversification. Stickleback evolvability is enhanced by standing genetic variation in the ancestral form (marine), but an important source of this variation is likely admixture between marine and older freshwater populations (Colosimo et al. 2005; Roesti et al. 2014). Admixture may also contribute to genetic variation in other fishes. For example, in many cichlid groups, including those from the African Great Lakes and Cameroon crater lakes, hybrid swarms may facilitate the onset of adaptive radiation (Stelkens et al. 2009; Meier et al. 2017; Irisarri et al. 2018; Poelstra et al. 2018) (see below). In San Salvador Island (Bahamas) pupfishes, adaptive introgression from a distant island 10 ka contributed to the divergent trophic morphology of specialists in the radiation, perhaps arising from a previous ephemeral radiation (Richards and Martin 2017). In Heliconius, introgression among lineages may lead to hybrid speciation (Heliconius Genome Consortium 2012) and, possibly, to more radiation (Merrill et al. 2015). Similarly, for the ancestral hawthorn-infesting population of Rhagoletis pomonella, part of the standing variation in diapause life history timing contributing to sympatric host shifts and speciation has an earlier history related to previous allopatric isolation, divergence, secondary contact, and admixture, beginning ~1.5 Ma that created latitudinal inversion clines (Feder et al. 2003). Admixture likely also contributed to evolvability in Galapagos finches (Lamichhaney et al. 2015; Chaves et al. 2016). However, other groups, including Hawaiian spiders (Cotoras et al. 2018) and honeycreepers (R. Fleischer, unpublished; Lerner et al. 2011; Knowlton et al. 2014) show little evidence of hybridization playing an ameliorative or other role.
The second part of this question (Supplementary Figure S1, VIIb) examined the extent to which disparity evolves repeatedly for lineages that occur in discrete areas (e.g., islands within an archipelago or a network of habitats across the landscape). Of the 26 responses, 15% considered that ecological disparity arose almost exclusively at the outset of the archipelago-wide radiation (species related across islands show niche conservatism). This was notable in Hawaiian Hyposmocoma moths (Haines et al. 2014), Hawaiian crab spiders (Garb and Gillespie 2009), Hawaiian Nesophrosyne leafhoppers (Bennett and O’Grady 2013), and Hawaiian honeycreepers (Lerner et al. 2011). In other lineages (58%), ecological disparity appears to have arisen repeatedly during the radiation. Here, diversification may occur in a replicated fashion (same ecological sets of taxa on each island/lake). This pattern is well known in the ecomorphs of Caribbean Anolis (Losos 2009), Tristan finches (Ryan et al. 2007), cichlids of the African Great Lakes (Muschick et al. 2012; Brawand et al. 2014), Hawaiian Tetragnatha spiders (Gillespie 2004), and Hawaiian Ariamnes spiders (Gillespie et al. 2018). Repeated evolution is also found among the ecotypes and ecomorphs of sticklebacks (Schluter and McPhail 1992; Rundle et al. 2000; Paccard et al. 2020) and the ecomorphs of Alpine whitefish (Vonlanthen et al. 2012). In still other lineages, the pattern of diversification between islands is unpredictable, notably in Cameroon crater-lake cichlids, Caribbean pupfishes, Galapagos land snails, and Hawaiian Metrosideros. Other responses included variations or combinations of these effects. In stickleback, for instance, predictability of divergence is highly dependent on spatial scale, being much higher on regional than global scales (Paccard et al. 2020).
On the basis of the responses above, we discuss generalities of how and when species diverge within a lineage that is undergoing adaptive radiation, in the context of (1) initial divergence, (2) persistence of reproductive isolation and achievement of local sympatry, and (3) admixture leading to exchange of adaptive traits among diversifying lineages.
Common Denominators—How Do Populations Within a Radiation Gain Reproductive Isolation
To initiate species formation in the course of adaptive radiation, generally a population must establish in new environmental conditions or in a new geographic location with the same environmental conditions (Mayr 1947; Coyne and Orr 2004), although there are exceptions (Hendry et al. 2009; Mallet et al. 2009; Feder et al. 2012; Hendry 2016). Reproductive isolation may develop quickly (Wheat et al. 2006), in particular when taxa that established some incompatibilities in allopatry, come together in sympatry (Coyne and Orr 1997); likewise, divergence may occur rapidly through ecological (Stuart et al. 2014; Dufour et al. 2017) or reproductive (Pfennig and Pfennig 2012a) character displacement. Given that speciation in sexually reproducing organisms involves the evolution of barriers to gene flow between populations, it is more likely to proceed when spatial, temporal or environmental separation restricts migration (Coyne and Orr 2004). Thus, it is important to consider how geographic barriers on the one hand and ecological shifts on the other hand facilitate species formation, including the time scale and relative order in which these arise, and their subsequent effect on the gene flow within and between populations.
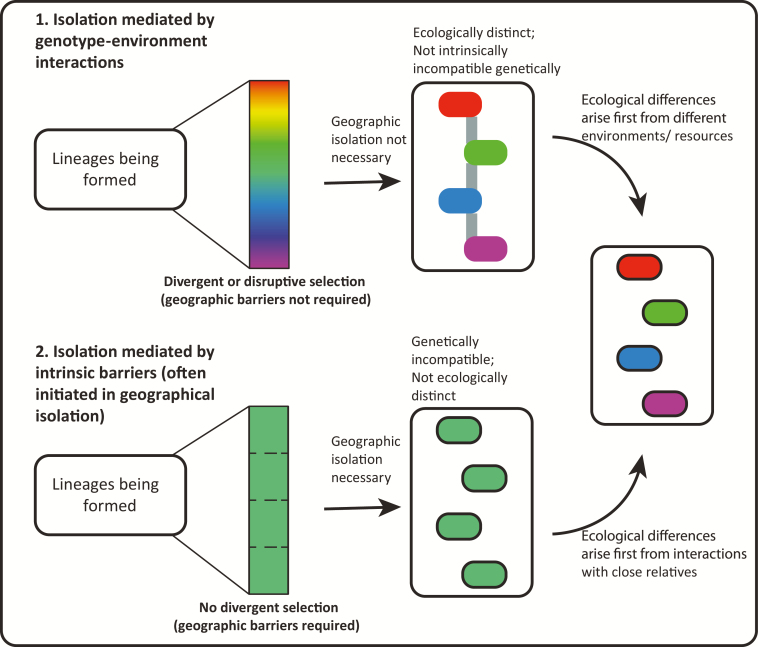
Initial separation of populations in an adaptive radiation may be achieved in different ways, and comparisons across radiations often fail to find commonalities. While the first step clearly requires the origin of a new population, and stable co-occurrence of sibling species requires a mechanism to overcome gene flow (Seehausen et al. 2014), initial divergence may or may not involve different ecological selection pressures (Mayr 1947;Fig. 2). Factors that drive initial divergence can be broken down into two broad categories relative to the radiation: external and internal. We define external factors as those that involve interactions with the environment external to the radiation membership (e.g., the physical environment or other unrelated species); external environmental effects tend to be coupled with divergent or disruptive selection (Figure 2.1). In other situations, genetic incompatibilities can arise without the need for interactions with the external environment and without external divergent selection (Figure 2.2), potentially linked to secondary sexual traits (Mendelson et al. 2014); subsequent ecological divergence (if it occurs) is likely associated with interaction among close relatives within the radiation (internal) (Brown and Wilson 1956; Pfennig and Pfennig 2010; Tilman and Snell-Rood 2014). The importance of these two mechanisms is also related to the rate of speciation and the degree and duration of geographic isolation, which we discuss later. Divergent sexual selection, depending on the specific mechanism and the role of the environment, may fit comfortably within either category.
Reproductive isolation coupled with divergent selection from the external environment
When initial reproductive isolation and ecological shifts are shaped by adaptation to the environment, speciation may proceed as a consequence of divergent selection in either the presence or absence of gene flow (Schluter 2001, 2009; Rundle and Nosil 2005; Nosil 2012) (Figure 2.1). For example, Galapagos wolf spiders (De Busschere et al. 2010) and beetles (Hendrickx et al. 2015) have repeatedly adapted to high and low elevation habitats. Similarly, host switching in parasites (Bush 1969; Price 1980; Feder et al. 1988; Drès and Mallet 2002; Forbes et al. 2009; Hood et al. 2015) or different pollinator communities (Schemske and Bradshaw 1999; Whittall and Hodges 2007) can generate new taxa via divergent ecological selection. This mechanism of initial divergence has been implicated in many other situations where populations respond to divergent selection in different environments, and can be accentuated by intraspecific competition within populations (Bolnick 2004; Levis et al. 2017). The very young lineages of sticklebacks and pupfish show strong evidence of divergent selection in the early phases of divergence (Schluter 2000; Hendry et al. 2009; Martin and Wainwright 2013), as do Rhagoletis, and other phytophagous insect specialists (Berlocher and Feder 2002). In each of these examples, the external environment leads to some kind of assortative mating (Richards et al. 2019); hence, species formation is explicitly tied to ecological differentiation.
Reproductive isolation coupled with geographic isolation
Isolated populations experiencing similar selective environments can evolve intrinsic genetic incompatibilities that arise by chance (Figure 2.2). When reproductive barriers are made up of intrinsic genetic incompatibilities, the taxa formed may be less prone to collapse or extinction than those arising from divergent selection alone (Seehausen 2006). Relative to extrinsic postzygotic or prezygotic incompatibilities that evolve under divergent selection (Seehausen et al. 2014), intrinsic incompatibilities that evolve by chance between populations may accrue at a slower rate (Price 2010). However, the rate at which intrinsic incompatibilities accumulate can be accelerated by parallel (non-divergent) selection as in speciation by “mutation-order” (Mani and Clarke 1990), where reproductive isolation evolves as a by-product of the fixation of different advantageous mutations between geographically isolated populations experiencing similar selection pressures (Schluter 2009). Moreover, population genetic models indicate that reproductive incompatibilities between populations initially experiencing similar natural and sexual selection can be amplified as a result of sexual traits (Agrawal et al. 2011; Mendelson et al. 2014): Secondary, sexual traits can fix differently in different populations that initially experience similar natural and sexual selection, with sexual preferences persisting even with low levels of gene flow (Mendelson et al. 2014). Such effects can lead to the rapid origins of ecologically similar taxa in allo- or parapatry (Rundell and Price 2009). Thus, species formation here is not explicitly tied to ecological differentiation. However, when sibling species come into contact, reproductive isolation may be accentuated rapidly due to reinforcement (Coyne and Orr 1997). Moreover, ecological differences can then arise through character displacement (Weber et al. 2017; Cotoras et al. 2018).
Which taxa are likely to diverge in which way?
In studies of reproductive isolation within an adaptive radiation, it can be difficult to distinguish the relative importance of reproductive isolation coupled with divergent selection from the external environment (Figure 2.1) versus reproductive isolation coupled with geographic isolation and without divergent selection where ecological differences may evolve later through character displacement (Figure 2.2). We often lack an adequate temporal framework over which to compare early stages with later stages of a radiation. Thus, in many of the classic examples of divergence of sympatric species pairs (e.g., stickleback (Schluter and McPhail 1992; Rundle et al. 2000; Boughman 2001) and Timema walking stick ecotypes (Nosil 2007)), the lineages are very young and diversity is low (single species pair). While these cases have allowed measuring selection at early stages of species divergence, in many of these cases it remains unknown whether one speciation event will lead to adaptive radiation of multiple co-occurring species (Glor 2010; Losos 2010; Stroud and Losos 2020), and what role ecological interactions among species within the radiation might eventually play in promoting or constraining further species and phenotypic diversification (Martin and Richards 2019).
As might be expected due to their often fine-tuned response of plants to local environmental conditions (Anacker and Strauss 2014), most plant radiations highlight the role of environmental factors external to the radiation and divergent ecological selection in the early stages of speciation; for example, despite exhibiting greater morphological and ecological diversity than the rest of the ~230 species in the genus distributed across five continents, divergence in Hawaiian Bidens appears to be driven by external factors in that all endemic species tested are cross-compatible, yet 70% of the 19 Hawaiian species are single-island endemics, and 85% are allopatric (or parapatric) when additionally considering habitat isolation within islands. Similarly, the numerous, predominantly intraspecific and co-occurring morphotypes of Hawaiian Metrosideros also show local adaptation to contrasting environments (e.g., Ekar et al. in review; Morrison and Stacy 2014), Sakishima et al., in prep.). Other plant radiations show a similar pattern of divergence between different environments, including silverswords and Schiedea in Hawaii, and various angiosperm clades in the Canary Islands (Gillespie and Baldwin 2010).
In contrast to divergent ecological or disruptive selection between environments (Maynard Smith 1966; Schluter 2009), resources, or hosts (Agrawal et al. 2011), there are multiple lineages in which initial reproductive isolation is coupled with geographic isolation and without divergent selection. The importance of isolation without divergent selection may be more pervasive in animals than plants (Anacker and Strauss 2014). The lack of divergent selection may lead to a necessity for more time needed for development of reproductive isolation (Price 2010). However, intrinsic reproductive barriers can develop more quickly when coupled with effects such as mutation order mediated by sexual selection (Mendelson et al. 2014). The role of geographic isolation without apparent divergent selection between ranges, has been demonstrated in Hawaiian spiders (Gillespie 2005; Cotoras et al. 2018), crickets, and flies (Hiller et al. 2019), as well as in planthoppers (Goodman et al. 2012). It has also been suggested for the early stages of divergence in Anolis lizards (Glor et al. 2003, 2004; Knouft et al. 2006; Stroud and Losos 2020) where diversification occurs within the same climatic niche (Wogan and Wang 2019), as well as in Galapagos snails (C. Parent, unpubl. data).
To conclude, the mechanism through which initial divergence is achieved during the course of an adaptive radiation varies considerably across radiations, depending on the role of divergent or disruptive selection in the initial divergence of populations (Mendelson et al. 2014). In many situations, especially in plants and taxa with tight associations to a resource, populations can diverge in response to selection that is divergent or disruptive and external to the radiation (Schluter 2001) (Figure 3). Alternatively, populations can diverge through adaptation to the same environment; in this case, ecological divergence—if it occurs—arises subsequently through interaction between relatives within the radiation (internal) (Rundell and Price 2009; Cotoras et al. 2018; Hiller et al. 2019) (Figure 4).
Figure 3.
Entities formed by factors external to the radiation membership and associated with divergent or disruptive selection (building on Figure 2, part 1). The external environmental conditions and divergent or disruptive selection can lead to reproductive isolation between descendant lineages, owing to genotype by environment interactions. In some lineages, tighter co-occurrence can be achieved through character displacement in secondary contact.
Figure 4.
Entities formed by reproductive incompatibility within the same environment—separation in geographical space without any obvious divergent or disruptive selection (building on Figure 2, part 2). Ecological divergence may arise through interaction with close relatives within the radiation subsequent to the development of reproductive incompatibilities.
Common Denominators—Persistence and Sympatry Within the Radiation
Genetic entities, whether distinct populations or incipient species, are formed continuously during adaptive radiation but most are likely to be ephemeral (Rosenblum et al. 2012). This is a general expectation from neutral theory, and not limited to adaptive radiations, as most species are expected to emanate from small local populations, which are then prone to extinction (Leigh 2007). Nevertheless, speciation rates estimated from the fossil record are much slower than those predicted both from mathematical models and empirical data from recent radiations (Seehausen et al. 2014). Thus, while speciation—or at least the formation of phenotypically distinct ecotypes—may be common and rapid in the context of adaptive radiation, most new entities may be short lived. Evolutionary studies should therefore focus on not only the formation of new species but also their persistence in space and time.
In the case of lineages that are in the very beginning stages of a radiation, many reproductively isolated ecotypes may form, but they tend to be eliminated by geological or climatological processes such as loss of lake environments (e.g., paleo-lake Makgadikgadi: (Joyce et al. 2005)), or by glaciation (e.g., stickleback and whitefish), or by ecological processes of predation and exclusion (e.g., stickleback (Gow et al. 2006; Taylor et al. 2006), Lake Victoria cichlids (Goldschmidt 1998; McGee et al. 2015) and Laguna Chichancanab pupfishes (Strecker 2006)). Another cause of nonpersistence of many species in adaptive radiations is that as long as reproductive isolation (and hence speciation) is only a consequence of divergent adaptation to alternative fitness optima or ecological niches, species will persist only as long as the fitness optima exist. Fitness landscapes can change with changes in the physical and biotic environment, and when such changes lead to the convergence of formerly distinct fitness peaks, the mechanism of reproductive isolation will no longer persist, and species will coalesce back into a single gene pool (although see discussion of the issue of population persistence above). Such speciation reversal has been described in adaptive radiations of cichlids (Seehausen et al. 1997), stickleback (Taylor et al. 2006), whitefish (Vonlanthen et al. 2012), and Darwin’s finches (Hendry et al. 2006; Kleindorfer et al. 2014), and it may be widespread in highly sympatric radiations in general.
A major question centers on the circumstances that lead to the persistence of entities as adaptive radiation proceeds and as the environmental theater changes. To get at this, we must first assess the hallmarks of adaptive radiation, notably the context of co-occurrence that allows species to accumulate, and at what scale (i.e., between sites or within sites). The geography of co-occurrence varies considerably among adaptive radiations: members of a radiation can occur in allopatry, parapatry, mosaic allopatry, or pure sympatry, including syntopy. By definition, allopatry, parapatry, and mosaic allopatry all imply some level of spatial (or temporal) separation of populations, while sympatry connotes extensive dispersal between populations (Mallet et al. 2009) or that individuals are physically capable of regular interaction (Mendelson and Shaw 2005; Weber et al. 2017). For the purpose of understanding adaptive radiation, a critical component is determining whether and how individuals of diverging populations interact when they are in proximity.
Entities shaped by the external environment and divergent selection
Divergent ecological selection can lead to reproductive isolation between descendant lineages, owing to genotype by environment interactions that disfavor intermediate ecological phenotypes (Figure 3). Such divergence may occur at various scales of geographic separation. For example, taxa may diverge across broad elevation zones (De Busschere et al. 2010) leading to sympatry at the island level but with limited interactions between ecotypes. Similarly, many sister species in adaptive radiations of fish in lakes and in the Sea diverge along water depth gradients, as has been shown for cichlid radiations, Alpine lake whitefish and Pacific Ocean rockfish (Seehausen and Wagner 2014). Some plants may differentiate based on fine-scale environmental heterogeneity (Anacker and Strauss 2014). In some fish, the tendency to specialize either on a littoral/benthic or a pelagic/limnetic life history gives rise to divergent selective pressures between juxtaposed habitat types. When sufficiently strong, or sufficiently strongly coupled to habitat structure, such divergent selection may sometimes lead to speciation without geographical isolation (Barluenga et al. 2006; Richards et al. 2019). However, in all of these situations, it is the external environment that plays the major role in shaping ecological and mating traits of the organism.
Maintenance of nascent species and secondary sympatry
For nascent species adapted to different environments, their maintenance as genetically distinct entities often, but not always, requires ongoing divergent selection, at least until genetically intrinsic reproductive incompatibilities accumulate (Calabrese and Pfennig 2020) (Figure 3.2). These nascent species will be vulnerable to ecological perturbations that disrupt the regimes of divergent selection and dispersal (Nosil et al. 2009). Thus, lineages formed through ecological speciation as a result of divergent selection between different external environments in parapatry, may be vulnerable to loss due to changes in selective regimes (Cutter and Gray 2016). The same would apply to cases of allopatric ecological speciation when changes in the selective regime coincide with a loss of a geographical barrier or change in dispersal regime. The temporal scales over which environments change and intrinsic incompatibilities become fixed within diverging populations are therefore important issues when reproductive isolation is initially based on such divergent selection.
Maintaining divergent selection
Based on the arguments above, it is likely that entities formed in the context of divergent selection between different external environments will tend to persist as long as the pressure imposed by divergent selection is maintained (Seehausen et al. 2014). When these divergent selection pressure, are due to differences in host, pollinator, or habitat fidelity where organisms preferentially choose to reside and mate in their natal habitats, positive assortative mating can emerge as a consequence of the interplay between habitat choice, mate choice, and performance. As a result, gene flow between habitats is reduced and population divergence accentuated in a process analogous to reinforcement except that further differentiation of habitat preference occurs rather than preference of mates (Thibert‐Plante and Gavrilets 2013). One example of this occurs in Rhagoletis flies that mate only on or near the fruit of their respective host plants and that use volatile compounds emitted from the surface of ripening fruit as key olfactory cues to discriminate among alternate hosts and mating arenas (Linn et al. 2003; Powell et al. 2012). If the divergent selection is strictly between spatially distinct environments, local (alpha) diversity of species cannot increase, but beta diversity may increase by increasing the spatial turnover as a result of increasingly tight associations with a given microenvironment, with mosaic or micro-allopatry (Figure 3.2). When the divergent selection is between microallopatric niches (such as host plants in Rhagoletis), the emerging species can be effectively sympatric at least for parts of their life cycle.
Order of events
The “habitat first rule” of adaptive radiation suggests that initial divergence often occurs as a consequence of environmental variability across space (Schluter 2000). A similar scenario has been suggested in a general vertebrate model (Streelman and Danley 2003).
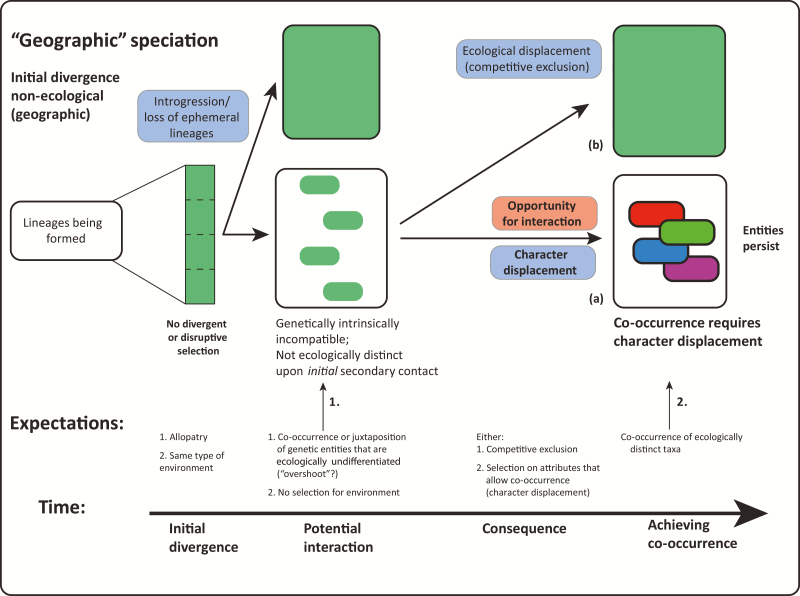
Entities Shaped by Intrinsic Reproductive Isolation and Ecological Divergence in Secondary Sympatry
The alternative to separation along the environmental/habitat boundary is separation in geographical space without any obvious divergent selection (Figure 4). In this case, populations, usually in similar environments, become isolated for a period of time (Figure 4.1), potentially sufficient to lead to the fixation of genetic incompatibilities as a result of genetic drift or parallel selection interacting with mutation order (Mendelson et al. 2014). Here again, after such isolation, taxa may or may not come back into contact.
Secondarily gaining local sympatry
First, interaction in local sympatry may be readily achieved for entities thus formed because the environments in which sister taxa have diverged are similar (Cotoras et al. 2018). Moreover, at least in some taxa, behavioral (prezygotic) reproductive isolation can be achieved upon secondary sympatry when sister taxa are already isolated by postzygotic incompatibilities; these events can precede the evolution of ecological or morphological differences (Orr 1995). Then, the expectation is that when ecologically similar but reproductively isolated taxa come together, competition for shared limiting resources will lead to ecological character displacement (Figure 4.2a) which stabilizes the coexistence of competing species in sympatry (Germain et al. 2018). Alternatively, competitive exclusion may lead to geographical disjunctions or extinction of one of the taxa (Figure 4.2b) (see section below). Of course, niche overlap can vary in space and time and, hence, species with broad niche overlap during much of the year can still coexist as long as they show substantial niche separation during critical periods (De León et al. 2014). Which of these outcomes occurs, when, and why some lineages are more prone to one or the other outcome of competition, is an open question.
Remaining in allopatry
Second, sibling taxa may remain in allopatry as in the classic form of nonadaptive radiation (Rundell and Price 2009) (i.e., remaining as shown in Figure 4.1) or they may persist in various forms of parapatry, microallopatry, or mosaic allopatry, but again without much local interaction.
Order of events
Unlike the “habitat first” model discussed above, when reproductive isolation occurs without any notable ecological shift, the first ecologically divergent traits to appear will be those associated with interactions arising from secondary sympatry of sibling species. This has been noted in a radiation of western North American Ceanothus (Ackerly et al. 2006), with traits that allow co-occurrence being the first axis of ecological divergence after complete allopatric speciation.
The arguments presented here come with many caveats because phylogenies cannot be used to reliably infer the geography of speciation (Losos and Glor 2003) and phylogenetic reconstructions are simply hypotheses, with inherent uncertainty. Without witnessing a temporal sequence of events, it is very difficult to test alternative hypotheses or to infer the role of extinction on these clade-level patterns. Some hotspot island archipelagoes or lakes that span a spectrum of ages have been used as temporal snapshots to reconstruct the evolutionary history of lineages (Shaw and Gillespie 2016), though here again, there are assumptions that taxa do not violate the temporal sequence (e.g., through “back colonization”).
To conclude, during adaptive radiation, when differentiation is tied to the external environment or habitat types (e.g., host or other associate), divergent selection between environments or hosts may often play the dominant role in shaping patterns of diversity (Figure 3). Persistence of nascent species, thus, generally requires ongoing divergent selection for alternate environments or associates. In a number of lineages, however, ecological divergence is achieved subsequent to geographic isolation, through direct interaction between close relatives internal to the radiation, leading to accumulation of local diversity through ecological character displacement (Figure 4).
Common Denominators—Isolation and Admixture
Early work on speciation stressed the importance of geographic isolation between populations, with mutation, genetic drift and indirect effects of natural selection causing speciation. It was generally believed that gene flow would counteract differentiation between populations, and research focused on isolating mechanisms that prevented gene flow (Merrell 1962). The rapidity of adaptive radiation in some systems then suggested various ways that differentiation could be achieved, with much attention focused on founder events and the possibility that premating isolating mechanisms could evolve quickly through sexual selection. Subsequent studies on Drosophila showed that both prezygotic and postzygotic reproductive isolation increase with divergence time between taxa, but secondary sympatry, or syntopy after extensive periods of allopatric divergence, has a very strong effect on increasing the rate at which prezygotic isolation can evolve, likely through selection for sexual recognition among genetically compatible genotypes (Coyne and Orr 1997). Various mechanisms have been proposed to explain elevated rates of divergence, including chromosomal rearrangements that can prevent recombination and allow genomic regions to diverge in the face of gene flow (Machado et al. 2007). While the importance of geographic isolation is widely accepted (Coyne and Orr 2004), occasional gene exchange may continue long after speciation, and quite often for species that are millions of years divergent (Grant and Grant 1992; Arnold 1997; Mallet 2005, 2008). Thus, speciation can occur without complete geographic isolation (Mallet 2008; Servedio and Noor 2003), in particular given sufficient divergent or disruptive selection and its association with mating habitat (Bush and Butlin 2004).
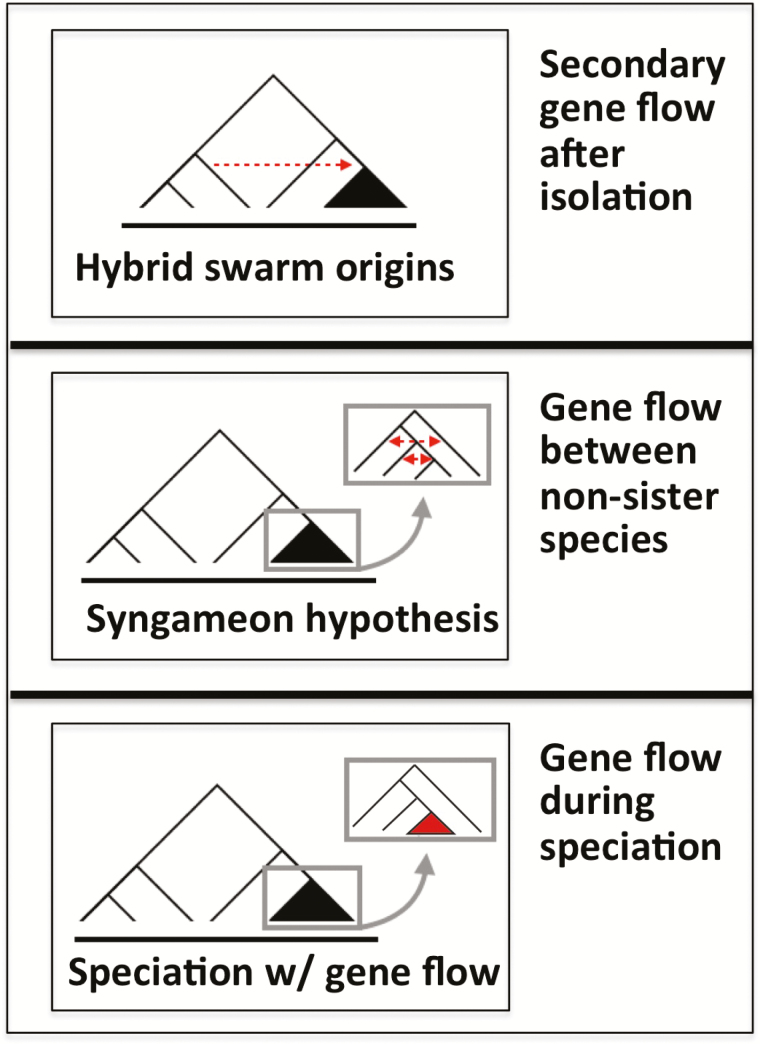
Given the above, it may initially come as a surprise that hybridization leading to genetic admixture may even facilitate adaptive radiation. Two distinct scenarios have been proposed (Seehausen 2004): 1) admixture occurring among nonsister species within/during an adaptive radiation may facilitate further speciation within the adaptive radiation, a concept known as the syngameon hypothesis (Seehausen 2004; Givnish 2010); and 2) admixture between distantly related species prior to adaptive radiation may facilitate the onset of adaptive radiation from the hybrid population, a concept referred to as hybrid swarm origins (Figure 5, Seehausen 2004). For ongoing speciation, gene flow between diverging populations will often stall further divergence. However, gene flow into one of two diverging populations from a third, more distantly related, population or species can allow the recruitment of alleles that may facilitate the divergence between the sister populations (Poelstra et al. 2018). Recent studies have started to focus on the genomic signatures and evolutionary consequences of admixture. When previously divergent populations come together, hybridization may lead to introgression which is genomically quantified as “admixture”. Its extent and genomic distribution depends on the degree and nature of genetic divergence between the entities involved prior to their contact, given the tendency for genetic incompatibilities to increase with time and genetic divergence (Matute et al. 2010). However, the frequency of phenotypic novelties that can arise spontaneously as a consequence of hybridization also tends to increase with time for divergence (Stelkens et al. 2009). Recent experimental work shows how both the genetic difference between hybridizing species and the number of species that contribute to a hybrid population affect the probability of reproductive isolation in the hybrid population. There appears to be a “sweet spot” between the minimum divergence necessary for the evolution of novel and advantageous recombinant genotypes and a maximum divergence, beyond which the accumulation of genetic incompatibilities eliminates any evolutionary impact of hybridization (Comeault and Matute 2018). These sweet spots of divergence prior to hybridization have the potential to play a key role in adaptive radiation, although the minimum and maximum divergence may differ greatly for different clades.
Figure 5.
Gene flow, traditionally considered to hinder divergence between incipient species, can serve to infuse variability that may foster adaptive radiation. This can take place through (a) hybrid origins of entire radiating clades (“hybrid swarm origins”) wherein admixture between one or more divergent lineages happens prior to the onset of radiation, (b) via hybridization between nonsister species within adaptive radiations that facilitates further speciation (“syngameon hypothesis”) (Seehausen 2004), and (c) speciation with gene flow between sister species. It is important to distinguish between how admixture is achieved in order to assess its effects on the process of adaptive radiation (Brock and Wagner 2018). Both syngameon and hybrid swarm origins hypotheses have now been well documented in cichlid fish (Meier et al. 2017), and the importance of gene flow and the syngameon have been well demonstrated in Heliconius butterflies (Mallet 2005; Merrill et al. 2015), as well as many plants (Barrier et al. 1999; Friar et al. 2008), and are also found in many other lineages (Feder et al. 2003; Lamichhaney et al. 2018). Indeed, the processes may be common to many adaptive radiations. Moreover, there may be a “sweet spot” in which divergent lineages can admix or hybridize and give rise to variability that is key to adaptive radiation.
Hybridization in the course of adaptive radiation sets up a scenario where gene flow and selection toward local adaptive peaks may interact. This will often happen between diverging sister taxa but it may also happen between more distantly related taxa within a radiation. Especially in the latter case, gene flow may introduce new combinations of genes that have never before been segregating in one population and may facilitate adaption or renewed speciation in the recipient population. Thus, occasional introgressive gene exchange between nonsister species in adaptive radiations may be important for construction of new gene and trait combinations in rapidly radiating taxa (Meier et al. 2017, 2018), in some cases leading to hybrid speciation (Lamichhaney et al. 2018). Hybridization has been well documented in a number of classic adaptive radiations including Hawaiian silverswords (Carr 1987; Carlquist et al. 2003), Hawaiian Bidens (Knope et al. 2020), Darwin’s finches in the Galapágos (Lamichhaney et al. 2015), Heliconius butterflies (Heliconius Genome Consortium 2012), and African cichlid fish (Seehausen 2015). However, demonstrating admixture among radiating species and demonstrating its effects on further adaptive radiation are two different things and whether admixture among members of a radiation actually enhances further speciation within adaptive radiation (Carr 1987) can be difficult to test. Testing the syngameon hypothesis of adaptive radiation therefore requires combining population genomic, demographic and phenotypic analyses (Meier et al. 2018).
The hybrid swarm origin of adaptive radiation is different from the syngameon hypothesis of adaptive radiation in that the onset of adaptive radiation happens in a population that is of hybrid origin between potentially quite distantly related species. Admixture between such species—that have not themselves diverged from each other under divergent natural selection but may have long history of completely independent evolution—introduces a wide range of genetic variants into a single population that have never cosegregated within a population. Such admixture between divergent taxa has been implicated in establishing the radiation of Hawaiian silverswords (Barrier et al. 1999), Rhagoletis fruit flies (Feder et al. 2003), and several cichlid radiations (Irisarri et al. 2018; Meier et al. 2017). The hybrid swarm origin hypothesis for adaptive radiation makes predictions that are unconfounded by the fact that species in young radiations tend to hybridize. Its unique predictions are, first, that the most recent common ancestor of all members of a radiation is a population of hybrid origin between distinct species, and second, that new combinations of old alleles brought together by the hybridization event (i.e., that did not exist in either of the parental lineages alone) play important roles in speciation and adaptation during the radiation (Seehausen 2004). This combination of hypotheses receives its strongest support to date from work on the Lake Victoria Region superflock of cichlid fish, which originated from hybridization between ecologically similar Astatotilapia/Thoracochromis species from the Upper Nile region and the upper Congo river, lineages which have diverged for millions of years in geographical isolation and are not very different ecologically (Meier et al. 2017). It is possible that variation in propensity for hybridization may help explain why some lineages radiate adaptively while other similar lineages do not (Meier et al. 2019). In smaller radiations of other cichlids and pupfish, there also is evidence that introgression from distantly related species outside the lake may have triggered adaptive radiation (Richards and Martin 2017; Poelstra et al. 2018; Richards et al. 2018). As our ability to test for these patterns with genomic data mounts, explicit tests of this hypothesis should become more common.
To conclude, mechanisms for the separation of gene pools into species are clearly required for adaptive radiation (Figure 5). However, it appears that genetic admixture between species may sometimes facilitate adaptive radiation, likely in conjunction with ecological opportunity and spatially heterogeneous or ecologically multifarious selection. However, as genomic evidence for admixture in the history of adaptive radiations increases, there is a need to carefully distinguish between the genomic signatures of processes associated with the hybrid swarm origin mechanism versus the syngameon mechanism of adaptive radiation. There is now clear genomic evidence for mechanisms associated with both hypotheses. Furthermore, these processes are both distinct from the commonly discussed speciation-with-gene flow, and caution is needed to avoid confounding the genomic signatures of these processes. Because the genetic and phenotypic novelty generated by hybridization tends to increase with the age of lineages while genetic incompatibilities increasingly prevent admixture of lineages when they are too divergent, there may be an optimal degree of divergence between populations or species at which admixture might facilitate adaptive radiation (Stelkens et al. 2010; Comeault and Matute 2018). The critical timing of admixture likely depends on attributes of the lineage in question, highlighting the need for comparative studies (Marques et al. 2019).
Conclusions
The most important outcome from the current assessment is that adaptive radiation can proceed along multiple distinct evolutionary trajectories. We can only make progress in developing a synthetic understanding of adaptive radiation and speciation if we can distinguish apples from oranges and understand both commonalities and differences broadly across different radiations. For example, in lineages in which initial divergence is linked to divergent selection between different environments external to the radiation, repeated evolution of ecotypes is a common outcome, such as those associated with high- and low-elevation wolf spiders in the Galapagos (De Busschere et al. 2010), adaptation to wet and dry habitats in Hawaiian silverswords (Blonder et al. 2016), and benthic and limnetic crater lake cichlids (Kusche et al. 2014) (Figure 3). In contrast, lineages of lizards and spiders are known for the repeated evolution of co-occurring and interacting species belonging to distinct ecomorphs (Losos 2009; Gillespie et al. 2018); this pattern may often be associated with ecological and/or reproductive character displacement due to interactions between closely related lineages that occur in secondary contact after a period of divergence in allopatry (Figure 4). Likewise, to evaluate the role of admixture in adaptive radiation, we must distinguish signatures consistent with the syngameon hypothesis from those consistent with the hybrid swarm hypothesis, and both from signatures expected under speciation-with-gene flow between incipient species (Figure 5). Once we recognize similarities and differences in the processes underlying diversification across a wide-range of lineages, we can look at other aspects of the evolutionary process upon which adaptive radiations can shed light, most notably concepts of specialization, abundance, and equilibrium diversity, to name a few. We have now an unprecedented array of tools to genetically dissect phenotypes, refine phylogenetic relationships and demographic histories, and more accurately resolve the tempo and mode of divergence.
However, at the same time we must also appreciate that many different processes are likely to interact and synergistically increase the biodiversity generated during adaptive radiation. Thus, while we may separate the factors driving specific aspects of species diversification, we must at the same time synthesize to fully comprehend the causal basis of adaptive radiations broadly. Moreover, in addition to elucidating how niches are filled, key questions remain concerning (i) how new or underutilized niches are created and (ii) whether and how adaptive radiation in one clade may reverberate throughout communities and ecosystems. Such cascading interactions (Brodersen et al. 2018) can potentially broadly influence biodiversity on multiple ecological scales. Given appropriate comparisons and a broad view of adaptive radiation, we have at our fingertips the ability to answer questions that have, until now, baffled our understanding of the diversification process. It is an exciting time for new and old investigators alike, as myriad research opportunities in adaptive radiation are now open and available to explore.
Glossary
Adaptive radiation—evolutionary divergence of members of a single phylogenetic lineage into a variety of different adaptive forms (Futuyma 1998).
Admixture—when individuals from two or more previously separated populations begin interbreeding resulting in the introduction of new genetic lineages into a population.
-
Character displacement
Reproductive character displacement is the adaptive evolution of traits that minimize deleterious reproductive interactions between species; when arising from selection to avoid hybridization, it is the same as reinforcement.
Ecological character displacement
Developmental plasticity—a developmental change in form or behavior caused by environmental conditions (West-Eberhard 2003).
Divergent selection—natural or sexual selection that favors different phenotypes in different populations.
Diversification—evolution of phenotypically and reproductively distinct species in a clade.
Disruptive selection—natural or sexual selection that favors extreme over intermediate phenotypes within a single population (Rueffler et al. 2006).
Ecological character displacement—the divergence of sympatric species to minimize ecological overlap.
Ecological opportunity—the availability of ecologically accessible resources that may be evolutionarily exploited (Stroud and Losos 2016).
Ecological speciation—the process by which barriers to gene flow evolve between populations as a result of ecologically based divergent selection (Schluter 2009).
Enemy-free space or release—‘‘ways of living that reduce or eliminate a species’’ vulnerability to one or more species of natural enemies’ including competitors (Jeffries and Lawton 1984).
Evolvability—the capacity to generate heritable phenotypic variation (Stroud and Losos 2016).
Hybrid swarm—a population of hybrids that has survived beyond the initial hybrid generation, with interbreeding between hybrid individuals and backcrossing with its parent types.
Introgression—gene flow from one species into the gene pool of another.
Key innovation—in evolution, any modification in structure or function that permits a lineage to exploit the environment in a more efficient or novel way and thereby creates ecological opportunity.
-
Geographic separation terms:
Allopatry: Separated in space in such a way as to prevent the organisms from interacting during normal activity.
Sympatry: No spatial separation, allowing organisms to interact regularly during normal activity.
Syntopy: a special case of sympatry involving the joint occurrence of two species in the same habitat at the same time.
Mosaic allopatry: Separated in space in a mosaic manner, i.e. with no single clear boundary, but the separation still preventing the organisms from interacting during normal activity.
Microallopatry: Despite overlapping range, microallopatric taxa are still separated in space with interaction between taxa prevented because they occupy different ecological niches.
Mutation-order speciation—different and incompatible mutations fix in different populations adapting to the same selective pressure (i.e. uniform selection, Mani and Clarke 1990). Mutations can arise stochastically in different order, allowing ‘mutation-order’ to serve as a stochastic driver of divergence (Nosil and Flaxman 2011).
Nonadaptive radiation—lineage diversification with minimal ecological diversification, resulting in allopatric or parapatric (Rundell and Price 2009) and sometimes even sympatric taxa (Xu and Shaw 2020).
Prezygotic barriers—anything that prevents mating and fertilization; postzygotic barriers—act after fertilization (zygote mortality, hybrid sterility)
-
Priority Effects (Fukami et al. 2007; De Meester et al. 2016):
Ecological priority effect: the arrival order of species influences community dynamics and structure.
Genetic priority effect: an evolutionary priority effect whereby the arrival order of genotypes influences population genetic structure.
Evolution-mediated priority effect: the arrival order of genetic lineages or species and their evolution influences population genetic or community dynamics.
Reinforcement—the evolution of traits that minimize hybridization between incipient species
-
Reproductive isolation—factors involved in initial stages
Extrinsic: Fitness reduction in hybrids that is dependent on the environment and that is mediated by genotype–environment interactions.
Intrinsic: often due to genetic incompatibilities; these occur independent of the environment (for example, Bateson–Dobzhansky–Muller incompatibilities).
Syngameon—Taxa that show long-term evidence of hybridization among multiple species (Lotsy 1925).
Taxon cycle—sequential phases of expansion and contraction of the ranges of species, associated generally with shifts in ecological distribution. The important contribution of the taxon cycle to adaptive radiation is its emphasis on evolutionary and ecological interactions among colonizing and resident species, which influence their extinction dynamics and establish patterns (Ricklefs and Bermingham 2002b).
Supplementary Material
Acknowledgements
We would like to thank the AGA 2018 for sponsoring the symposium on the “Origins of Adaptive Radiation” held in Waimea, Hawaii, July 2018, and we thank all the participants at the meeting for lively and creative discussions. Particular thanks to Anjanette Baker for her outstanding efforts in allowing the symposium to run smoothly, and to Bill Murphy for his help with the manuscript. The manuscript was much improved by thoughtful and extensive suggestions from Egbert Leigh, George Roderick, and one anonymous reviewer.
References
- Ackerly DD, Schwilk DW, Webb CO. 2006. Niche evolution and adaptive radiation: testing the order of trait divergence. Ecology. 87:S50–S61. [DOI] [PubMed] [Google Scholar]
- Agrawal AF, Feder JL, Nosil P. 2011. Ecological divergence and the origins of intrinsic postmating isolation with gene flow. Int J Ecol. 2011. [Google Scholar]
- Allender CJ, Seehausen O, Knight ME, Turner GF, Maclean N. 2003. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proc Natl Acad Sci USA. 100:14074–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker BL, Strauss SY. 2014. The geography and ecology of plant speciation: range overlap and niche divergence in sister species. Proc Biol Sci. 281:20132980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast BS, Drovetski SV, Curry RL, Boag PT, Seutin G, Grant PR, Grant BR, Anderson DJ. 2006. The origin and diversification of Galapagos mockingbirds. Evolution. 60:370–382. [PubMed] [Google Scholar]
- Arnold ML 1997. Natural hybridization and evolution. Oxford: Oxford University Press. [Google Scholar]
- Barluenga M, Stölting KN, Salzburger W, Muschick M, Meyer A. 2006. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature. 439:719–723. [DOI] [PubMed] [Google Scholar]
- Barrier M, Baldwin BG, Robichaux RH, Purugganan MD. 1999. Interspecific hybrid ancestry of a plant adaptive radiation: allopolyploidy of the Hawaiian silversword alliance (Asteraceae) inferred from floral homeotic gene duplications. Mol Biol Evol. 16:1105–1113. [DOI] [PubMed] [Google Scholar]
- Beheregaray LB, Gibbs JP, Havill N, Fritts TH, Powell JR, Caccone A. 2004. Giant tortoises are not so slow: rapid diversification and biogeographic consensus in the Galápagos. Proc Natl Acad Sci USA. 101:6514–6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GM, Moran NA. 2015. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci USA. 112:10169–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GM, O’Grady PM. 2013. Historical biogeography and ecological opportunity in the adaptive radiation of native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne). J Biogeogr. 40:1512–1523. [Google Scholar]
- Bennett GM, O’Grady PM. 2012. Host-plants shape insect diversity: phylogeny, origin, and species diversity of native Hawaiian leafhoppers (Cicadellidae: Nesophrosyne). Mol Phylogenet Evol. 65:705–717. [DOI] [PubMed] [Google Scholar]
- Berlocher SH, Feder JL. 2002. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu Rev Entomol. 47:773–815. [DOI] [PubMed] [Google Scholar]
- Blonder B, Baldwin BG, Enquist BJ, Robichaux RH. 2016. Variation and macroevolution in leaf functional traits in the Hawaiian silversword alliance (Asteraceae). J Ecol. 104:219–228. [Google Scholar]
- Bolnick DI 2004. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 58:608–618. [PubMed] [Google Scholar]
- Bolnick DI 2011. Sympatric speciation in threespine stickleback: why not? Int J Ecol. 2011 942847. doi:10.1155/2011/942847. [Google Scholar]
- Boughman JW 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature. 411:944–948. [DOI] [PubMed] [Google Scholar]
- Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, Simakov O, Ng AY, Lim ZW, Bezault E, et al. . 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 513:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock CD, Wagner CE. 2018. The smelly path to sympatric speciation? Mol Ecol. 27:4153–4156. [DOI] [PubMed] [Google Scholar]
- Brodersen J, Post DM, Seehausen O. 2018. Upward adaptive radiation cascades: predator diversification induced by prey diversification. Trends Ecol Evol. 33:59–70. [DOI] [PubMed] [Google Scholar]
- Brown WL, Wilson EO. 1956. Character displacement. Systematic Zool. 5:49–64. [Google Scholar]
- Bürger R, Schneider KA, Willensdorfer M. 2006. The conditions for speciation through intraspecific competition. Evolution. 60:2185–2206. [PubMed] [Google Scholar]
- Burns MD, Sidlauskas BL. 2019. Ancient and contingent body shape diversification in a hyperdiverse continental fish radiation. Evolution. 73:569–587. [DOI] [PubMed] [Google Scholar]
- Bush GL 1969. Sympatric host race formation and speciation in frugivorous flies of the genus rhagoletis (Diptera, Tephritidae). Evolution. 23:237–251. [DOI] [PubMed] [Google Scholar]
- Bush GL, Butlin RK. 2004. Sympatric speciation in insects. Adaptive Speciation, 229–248. [Google Scholar]
- Calabrese G, Pfennig K. 2020. Reinforcement and the proliferation of species. J Hered. 111:138–146. [DOI] [PubMed] [Google Scholar]
- Campana MG, Corvelo A, Shelton J, et al. . 2020. Adaptive radiation genomics of two ecologically divergent Hawai‘ian honeycreepers: the ‘akiapōlā‘au and the Hawai‘i ‘amakihi. J Heredity. 111:21–31. [DOI] [PubMed] [Google Scholar]
- Carlquist S, Baldwin BG, Carr GD. 2003. Tarweeds and silverswords. evolution of the Madiinae (Asteraceae). St. Louis, Missouri: Missouri Botanical Garden Press. [Google Scholar]
- Carr GD 1987. Beggar’s ticks and tarweeds: masters of adaptive radiation. Trends Ecol Evol. 2:192–195. [DOI] [PubMed] [Google Scholar]
- Chaves JA, Cooper EA, Hendry AP, Podos J, De León LF, Raeymaekers JA, MacMillan WO, Uy JA. 2016. Genomic variation at the tips of the adaptive radiation of Darwin’s finches. Mol Ecol. 25:5282–5295. [DOI] [PubMed] [Google Scholar]
- Chen Z-Q, Benton MJ. 2012. The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat Geosci. 5:375–383. [Google Scholar]
- Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G Jr, Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 307:1928–1933. [DOI] [PubMed] [Google Scholar]
- Comeault AA, Matute DR. 2018. Genetic divergence and the number of hybridizing species affect the path to homoploid hybrid speciation. Proc Natl Acad Sci USA. 115:9761–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell HV 2013. Is regional species diversity bounded or unbounded? Biol Rev Camb Philos Soc. 88:140–165. [DOI] [PubMed] [Google Scholar]
- Cotoras DD, Bi K, Brewer MS, Lindberg DR, Prost S, Gillespie RG. 2018. Co-occurrence of ecologically similar species of Hawaiian spiders reveals critical early phase of adaptive radiation. BMC Evol Biol. 18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J, Orr H. 2004. Speciation. Sunderland, MA: Sinauer. [Google Scholar]
- Coyne JA, Orr HA. 1997. “Patterns of speciation in Drosophila” revisited. Evolution. 51:295–303. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Gray JC. 2016. Ephemeral ecological speciation and the latitudinal biodiversity gradient. Evolution. 70:2171–2185. [DOI] [PubMed] [Google Scholar]
- Czekanski-Moir JE, Rundell RJ. 2019. The ecology of nonecological speciation and nonadaptive radiations. Trends Ecol Evol. 34:400–415. [DOI] [PubMed] [Google Scholar]
- Dambroski HR, Feder JL. 2007. Host plant and latitude-related diapause variation in Rhagoletis pomonella: a test for multifaceted life history adaptation on different stages of diapause development. J Evol Biol. 20:2101–2112. [DOI] [PubMed] [Google Scholar]
- Dawson J, Stemmermann R. 1990. Metrosideros (Gaud). In: Wagner WL, Herbst DR, Sohmer SH, editors. Manual of the flowering plants of Hawaii. Honolulu, Hawai’i: Univ. Hawai’i Press; p. 964–970. [Google Scholar]
- De Busschere C, Hendrickx F, Van Belleghem SM, Backeljau T, Lens L, Baert L. 2010. Parallel habitat specialization within the wolf spider genus Hogna from the Galápagos. Mol Ecol. 19:4029–4045. [DOI] [PubMed] [Google Scholar]
- De León LF, Podos J, Gardezi T, Herrel A, Hendry AP. 2014. Darwin’s finches and their diet niches: the sympatric coexistence of imperfect generalists. J Evol Biol. 27:1093–1104. [DOI] [PubMed] [Google Scholar]
- De Meester L, Vanoverbeke J, Kilsdonk LJ, Urban MC. 2016. Evolving perspectives on monopolization and priority effects. Trends Ecol Evol. 31:136–146. [DOI] [PubMed] [Google Scholar]
- Doellman MM, Egan SP, Ragland GJ, Meyers PJ, Hood GR, Powell THQ, Lazorchak P, Hahn DA, Berlocher SH, Nosil P, et al. . 2019. Standing geographic variation in eclosion time and the genomics of host race formation in Rhagoletis pomonella fruit flies. Ecol Evol. 9:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ, Sanderson MJ. 2015. Confluence, synnovation, and depauperons in plant diversification. New Phytol. 207:260–274. [DOI] [PubMed] [Google Scholar]
- Drès M, Mallet J. 2002. Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc Lond B Biol Sci. 357:471–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour CM, Herrel A, Losos JB. 2017. Ecological character displacement between a native and an introduced species: the invasion of Anolis cristatellus in Dominica. Biol J Linnean Soc. 123:43–54. [Google Scholar]
- Ehrlich PR, Raven PH. 1964. Butterflies and plants: a study in coevolution. Evolution 18:586–608. [Google Scholar]
- Ekar JM, Price DK, Johnson MA, Stacy EA. 2019. Varieties of the highly dispersible and hypervariable tree, Metrosideros polymorpha, differ in response to mechanical stress and light across a sharp ecotone. Am J Bot. 106:1106–1115. [DOI] [PubMed] [Google Scholar]
- Emerson BC, Gillespie RG. 2008. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol Evol. 23:619–630. [DOI] [PubMed] [Google Scholar]
- Feder JL, Berlocher SH, Roethele JB, Dambroski H, Smith JJ, Perry WL, Gavrilovic V, Filchak KE, Rull J, Aluja M. 2003. Allopatric genetic origins for sympatric host-plant shifts and race formation in Rhagoletis. Proc Natl Acad Sci USA. 100:10314–10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JL, Chilcote CA, Bush GL. 1988. Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature (London) 336:61–64. [Google Scholar]
- Feder JL, Egan SP, Nosil P. 2012. The genomics of speciation-with-gene-flow. Trends Genet. 28:342–350. [DOI] [PubMed] [Google Scholar]
- Feduccia A 2003. ‘Big Bang’ for Tertiary birds? Trends Ecol Evol. 18:172–176. [Google Scholar]
- Forbes AA, Powell TH, Stelinski LL, Smith JJ, Feder JL. 2009. Sequential sympatric speciation across trophic levels. Science. 323:776–779. [DOI] [PubMed] [Google Scholar]
- Friar EA, Prince LM, Cruse-Sanders JM, et al. . 2008. Hybrid origin and genomic mosaicism of Dubautia scabra (Hawaiian silversword alliance; Asteraceae, Madiinae). Systematic Bot. 33:589–597. [Google Scholar]
- Fukami T 2015. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Systematics. 46:1–23. [Google Scholar]
- Fukami T, Beaumont HJ, Zhang XX, Rainey PB. 2007. Immigration history controls diversification in experimental adaptive radiation. Nature. 446:436–439. [DOI] [PubMed] [Google Scholar]
- Futuyma DJ 1987. On the role of species in anagenesis. Am Nat. 130:465–473. [Google Scholar]
- Futuyma DJ 1998. Evolutionary biology, 3rd edn Sunderland, MA: Sinauer. [Google Scholar]
- Ganders F, Nagata K. 1984. The role of hybridization in the evolution of Bidens on the Hawaiian Islands. In: Grant WF, editor. Plant biosystematics. Toronto: Academic Press, p. 179–194. [Google Scholar]
- Garb JE, Gillespie RG. 2009. Diversity despite dispersal: colonization history and phylogeography of Hawaiian crab spiders inferred from multilocus genetic data. Mol Ecol. 18:1746–1764. [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Losos JB. 2009. Adaptive radiation: contrasting theory with data. Science. 323:732–737. [DOI] [PubMed] [Google Scholar]
- Germain RM, Williams JL, Schluter D, Angert AL. 2018. Moving character displacement beyond characters using contemporary coexistence theory. Trends Ecol Evol. 33:74–84. [DOI] [PubMed] [Google Scholar]
- Gillespie R 2004. Community assembly through adaptive radiation in Hawaiian spiders. Science. 303:356–359. [DOI] [PubMed] [Google Scholar]
- Gillespie RG 2005. Geographical context of speciation in a radiation of Hawaiian Tetragnatha spiders (Araneae, Tetragnathidae). J Arachnol. 33:313–322. [Google Scholar]
- Gillespie RG 2016. Island time and the interplay between ecology and evolution in species diversification. Evol Appl. 9:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RG, Baldwin BG. 2010. Island biogeography of remote archipelagoes. The theory of island biogeography revisited, 358–387. [Google Scholar]
- Gillespie RG, Benjamin SP, Brewer MS, Rivera MAJ, Roderick GK. 2018. Repeated diversification of ecomorphs in Hawaiian stick spiders. Curr Biol. 28:941–947.e3. [DOI] [PubMed] [Google Scholar]
- Gillespie RG, Howarth FG, Roderick GK. 2001. Adaptive radiation. In: Levin SA editor. Encyclopedia of biodiversity. New York: Academic Press, p. 25–44. [Google Scholar]
- Gittenberger E 1991. What about non-adaptive radiation? Biol J Linnean Soc. 43:263–272. [Google Scholar]
- Givnish TJ 2010. Ecology of plant speciation. Taxon 59:1326–1366. [Google Scholar]
- Givnish TJ 2015. Adaptive radiation versus ‘radiation’ and ‘explosive diversification’: why conceptual distinctions are fundamental to understanding evolution. New Phytol. 207:297–303. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Sytsma KJ. 1997. Molecular evolution and adaptive radiation. Cambridge: Cambridge University Press. [Google Scholar]
- Glor RE 2010. Phylogenetic insights on adaptive radiation. Annu Rev Ecol Evol Systematics. 41:251–270. [Google Scholar]
- Glor RE, Gifford ME, Larson A, Losos JB, Schettino LR, Chamizo Lara AR, Jackman TR. 2004. Partial island submergence and speciation in an adaptive radiation: a multilocus analysis of the Cuban green anoles. Proc Biol Sci. 271:2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glor RE, Kolbe JJ, Powell R, Larson A, Losos J. 2003. Phylogenetic analysis of ecological and morphological diversification in Hispaniolan trunk-ground anoles (Anolis cybotes group). Evolution. 57:2383–2397. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T 1998. Darwin’s dreampond: drama in Lake Victoria. Cambridge (MA): MIT Press. [Google Scholar]
- Goodman KR, Welter SC, Roderick GK. 2012. Genetic divergence is decoupled from ecological diversification in the Hawaiian Nesosydne planthoppers. Evolution. 66:2798–2814. [DOI] [PubMed] [Google Scholar]
- Gow JL, Peichel CL, Taylor EB. 2006. Contrasting hybridization rates between sympatric three-spined sticklebacks highlight the fragility of reproductive barriers between evolutionarily young species. Mol Ecol. 15:739–752. [DOI] [PubMed] [Google Scholar]
- Grant PR 1999. Ecology and evolution of Darwin’s finches. Princeton (NJ): Princeton University Press. [Google Scholar]
- Grant PR, Grant BR. 1992. Hybridization of bird species. Science. 256:193–197. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. 2014. 40 years of evolution: Darwin’s finches on Daphne Major Island. Princeton (NJ): Princeton University Press. [Google Scholar]
- Haines WP, Schmitz P, Rubinoff D. 2014. Ancient diversification of Hyposmocoma moths in Hawaii. Nat Commun. 5:3502. [DOI] [PubMed] [Google Scholar]
- Harmon LJ, Losos JB, Jonathan Davies T, Gillespie RG, Gittleman JL, Bryan Jennings W, Kozak KH, McPeek MA, Moreno-Roark F, Near TJ, et al. . 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution. 64:2385–2396. [DOI] [PubMed] [Google Scholar]
- Heliconius Genome Consortium 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature (London) 487:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx F, Backeljau T, Dekoninck W, Van Belleghem SM, Vandomme V, Vangestel C. 2015. Persistent inter- and intraspecific gene exchange within a parallel radiation of caterpillar hunter beetles (Calosoma sp.) from the Galápagos. Mol Ecol. 24:3107–3121. [DOI] [PubMed] [Google Scholar]
- Hendry AP 2016. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- Hendry AP, Bolnick DI, Berner D, Peichel CL. 2009. Along the speciation continuum in sticklebacks. J Fish Biol. 75:2000–2036. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Grant PR, Rosemary Grant B, Ford HA, Brewer MJ, Podos J. 2006. Possible human impacts on adaptive radiation: beak size bimodality in Darwin’s finches. Proc Biol Sci. 273:1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Huber SK, De León LF, Herrel A, Podos J. 2008. Disruptive selection in a bimodal population of Darwin’s finches. Proc Biol Sci. 276:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller A, Koo M, Goodman K, et al. . 2019. Niche conservatism predominates in adaptive radiation: comparing the diversification of Hawaiian arthropods using Ecological Niche Modeling. Biol J Linnean Soc. 127: 479–492. [Google Scholar]
- Hood GR, Forbes AA, Powell TH, Egan SP, Hamerlinck G, Smith JJ, Feder JL. 2015. Sequential divergence and the multiplicative origin of community diversity. Proc Natl Acad Sci USA. 112:E5980–E5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormiga G, Arnedo M, Gillespie RG. 2003. Speciation on a conveyor belt: sequential colonization of the Hawaiian islands by Orsonwelles spiders (Araneae, Linyphiidae). Syst Biol. 52:70–88. [DOI] [PubMed] [Google Scholar]
- Hudson AG, Vonlanthen P, Seehausen O. 2011. Rapid parallel adaptive radiations from a single hybridogenic ancestral population. Proc Biol Sci. 278:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull P 2015. Life in the Aftermath of mass extinctions. Curr Biol. 25:R941–R952. [DOI] [PubMed] [Google Scholar]
- Irisarri I, Singh P, Koblmüller S, Torres-Dowdall J, Henning F, Franchini P, Fischer C, Lemmon AR, Lemmon EM, Thallinger GG, et al. . 2018. Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes. Nat Commun. 9:3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries M, Lawton J. 1984. Enemy free space and the structure of ecological communities. Biol J Linnean Soc. 23:269–286. [Google Scholar]
- Joyce DA, Lunt DH, Bills R, Turner GF, Katongo C, Duftner N, Sturmbauer C, Seehausen O. 2005. An extant cichlid fish radiation emerged in an extinct Pleistocene lake. Nature. 435:90–95. [DOI] [PubMed] [Google Scholar]
- Kleindorfer S, O’Connor JA, Dudaniec RY, Myers SA, Robertson J, Sulloway FJ. 2014. Species collapse via hybridization in Darwin’s tree finches. Am Nat. 183:325–341. [DOI] [PubMed] [Google Scholar]
- Knope ML, Bellinger MR, Datlof EM, Gallaher TJ, Johnson MA. 2020. Insights into the evolutionary history of the Hawaiian Bidens (Asteraceae) adaptive radiation revealed through phylogenomics. J Hered. 111:119–137. [DOI] [PubMed] [Google Scholar]
- Knope ML, Morden CW, Funk VA, Fukami T. 2012. Area and the rapid radiation of Hawaiian Bidens (Asteraceae). J Biogeogr. 39:1206–1216. [Google Scholar]
- Knope ML, Pender RJ, Crawford DJ, Wieczorek AM. 2013. Invasive congeners are unlikely to hybridize with native Hawaiian Bidens (Asteraceae). Am J Bot. 100:1221–1226. [DOI] [PubMed] [Google Scholar]
- Knouft JH, Losos JB, Glor RE, Kolbe JJ. 2006. Phylogenetic analysis of the evolution of the niche in lizards of the Anolis sagrei group. Ecology. 87:S29–S38. [DOI] [PubMed] [Google Scholar]
- Knowlton JL, Flaspohler DJ, Mcinerney NR, Fleischer RC. 2014. First record of hybridization in the Hawaiian Honeycreepers:’I’iwi (Vestiaria coccinea)×’Apapane (Himatione sanguinea). Wilson J Ornithol. 126:562–568. [Google Scholar]
- Knudsen R, Primicerio R, Amundsen PA, Klemetsen A. 2010. Temporal stability of individual feeding specialization may promote speciation. J Anim Ecol. 79:161–168. [DOI] [PubMed] [Google Scholar]
- Kozak KH, Blaine RA, Larson A. 2006. Gene lineages and eastern North American palaeodrainage basins: phylogeography and speciation in salamanders of the Eurycea bislineata species complex. Mol Ecol. 15:191–207. [DOI] [PubMed] [Google Scholar]
- Kozak KH, Wiens JJ. 2010. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am Nat. 176:40–54. [DOI] [PubMed] [Google Scholar]
- Kusche H, Recknagel H, Elmer KR, Meyer A. 2014. Crater lake cichlids individually specialize along the benthic-limnetic axis. Ecol Evol. 4:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhaney S, Berglund J, Almén MS, Maqbool K, Grabherr M, Martinez-Barrio A, Promerová M, Rubin CJ, Wang C, Zamani N, et al. . 2015. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature. 518:371–375. [DOI] [PubMed] [Google Scholar]
- Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR. 2018. Rapid hybrid speciation in Darwin’s finches. Science. 359:224–228. [DOI] [PubMed] [Google Scholar]
- Leigh EG Jr 2007. Neutral theory: a historical perspective. J Evol Biol. 20:2075–2091. [DOI] [PubMed] [Google Scholar]
- Leigh EG, Hladik A, Hladik CM, Jolly A. 2007. The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play? Rev Ecol. 62:105–168. [Google Scholar]
- Lerner HR, Meyer M, James HF, Hofreiter M, Fleischer RC. 2011. Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian honeycreepers. Curr Biol. 21:1838–1844. [DOI] [PubMed] [Google Scholar]
- Levis NA, Martin RA, O’Donnell KA, Pfennig DW. 2017. Intraspecific adaptive radiation: competition, ecological opportunity, and phenotypic diversification within species. Evolution. 71:2496–2509. [DOI] [PubMed] [Google Scholar]
- Linn C Jr, Feder JL, Nojima S, Dambroski HR, Berlocher SH, Roelofs W. 2003. Fruit odor discrimination and sympatric host race formation in Rhagoletis. Proc Natl Acad Sci USA. 100:11490–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, CA: University of California Press. [Google Scholar]
- Losos JB 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. American Society of Naturalists E. O. Wilson award address. Am Nat. 175:623–639. [DOI] [PubMed] [Google Scholar]
- Losos JB, Glor RE. 2003. Phylogenetic comparative methods and the geography of speciation. Trends Ecol Evol. 18:220–227. [Google Scholar]
- Lotsy J 1925. Species or linneon. Genetica 7:487–506. [Google Scholar]
- Maan ME, Seehausen O. 2011. Ecology, sexual selection and speciation. Ecol Lett. 14:591–602. [DOI] [PubMed] [Google Scholar]
- Machado CA, Haselkorn TS, Noor MA. 2007. Evaluation of the genomic extent of effects of fixed inversion differences on intraspecific variation and interspecific gene flow in Drosophila pseudoobscura and D. persimilis. Genetics. 175:1289–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnacca KN, Price DK. 2015. Rapid adaptive radiation and host plant conservation in the Hawaiian picture wing Drosophila (Diptera: Drosophilidae). Mol Phylogenet Evol. 92:226–242. [DOI] [PubMed] [Google Scholar]
- Mallet J 2005. Hybridization as an invasion of the genome. Trends Ecol Evol. 20:229–237. [DOI] [PubMed] [Google Scholar]
- Mallet J 2008. Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Philos Trans R Soc Lond B Biol Sci. 363:2971–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J, Meyer A, Nosil P, Feder JL. 2009. Space, sympatry and speciation. J Evol Biol. 22:2332–2341. [DOI] [PubMed] [Google Scholar]
- Mani GS, Clarke BC. 1990. Mutational order: a major stochastic process in evolution. Proc R Soc Lond B Biol Sci. 240:29–37. [DOI] [PubMed] [Google Scholar]
- Marques DA, Meier JI, Seehausen O. 2019. A combinatorial view on speciation and adaptive radiation. Trends Ecol Evol. 34:531–544. [DOI] [PubMed] [Google Scholar]
- Martin CH 2012. Weak disruptive selection and incomplete phenotypic divergence in two classic examples of sympatric speciation: Cameroon crater lake cichlids. Am Nat. 180:E90–E109. [DOI] [PubMed] [Google Scholar]
- Martin CH, Richards EJ. 2019. The paradox behind the pattern of rapid adaptive radiation: how can the speciation process sustain itself through an early burst? Annu Rev Ecol Evolut Systematics. 50:569–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC. 2013. On the measurement of ecological novelty: scale-eating pupfish are separated by 168 my from other scale-eating fishes. PLoS One. 8:e71164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matessi C, Gimelfarb A, Gavrilets S. 2002. Long-term build up of reproductive isolation promoted by disruptive selection: how far does it go? Selection 2:41–64. [Google Scholar]
- Matute DR, Butler IA, Turissini DA, Coyne JA. 2010. A test of the snowball theory for the rate of evolution of hybrid incompatibilities. Science. 329:1518–1521. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J 1966. Sympatric speciation. Am Nat. 100:637–650. [Google Scholar]
- Mayr E 1947. Ecological factors in speciation. Evolution 1:263–288. [Google Scholar]
- McGee MD, Borstein SR, Neches RY, Buescher HH, Seehausen O, Wainwright PC. 2015. A pharyngeal jaw evolutionary innovation facilitated extinction in Lake Victoria cichlids. Science. 350:1077–1079. [DOI] [PubMed] [Google Scholar]
- Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat Commun. 8:14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JI, Marques DA, Wagner CE, Excoffier L, Seehausen O. 2018. Genomics of parallel ecological speciation in Lake Victoria Cichlids. Mol Biol Evol. 35:1489–1506. [DOI] [PubMed] [Google Scholar]
- Meier JI, Stelkens R, Joyce DA, et al. . 2019. The coincidence of ecological opportunity with hybridization explains the prevalence of rapid adaptive radiation. Nature Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson TC, Martin MD, Flaxman SM. 2014. Mutation-order divergence by sexual selection: diversification of sexual signals in similar environments as a first step in speciation. Ecol Lett. 17:1053–1066. [DOI] [PubMed] [Google Scholar]
- Mendelson TC, Shaw KL. 2005. Sexual behaviour: rapid speciation in an arthropod. Nature. 433:375–376. [DOI] [PubMed] [Google Scholar]
- Merrell DJ. 1962. Evolution and Genetics: The Modern Theory of Evolution. New York (NY): Holt, Rinehart and Winston. [Google Scholar]
- Merrill RM, Dasmahapatra KK, Davey JW, Dell’Aglio DD, Hanly JJ, Huber B, Jiggins CD, Joron M, Kozak KM, Llaurens V, et al. . 2015. The diversification of Heliconius butterflies: what have we learned in 150 years? J Evol Biol. 28:1417–1438. [DOI] [PubMed] [Google Scholar]
- Morlon H, Parsons TL, Plotkin JB. 2011. Reconciling molecular phylogenies with the fossil record. Proc Natl Acad Sci U S A. 108:16327–16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KR, Stacy EA. 2014. Intraspecific divergence and evolution of a life-history trade-off along a successional gradient in Hawaii’s Metrosideros polymorpha. J Evol Biol. 27:1192–1204. [DOI] [PubMed] [Google Scholar]
- Moser FN, van Rijssel JC, Mwaiko S, et al. . 2018. The onset of ecological diversification 50 years after colonization of a crater lake by haplochromine cichlid fishes. Proc Roy Soc B: Biol Sci. 285:20180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Clark B, Johnson MS. 1993. Adaptive radiation and community structure of Partula on Moorea. Proc Roy Soc Lond. Series B: Biol Sci. 254:205–211. [Google Scholar]
- Muschick M, Indermaur A, Salzburger W. 2012. Convergent evolution within an adaptive radiation of cichlid fishes. Curr Biol. 22:2362–2368. [DOI] [PubMed] [Google Scholar]
- Nosil P 2007. Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Am Nat. 169:151–162. [DOI] [PubMed] [Google Scholar]
- Nosil P 2012. Ecological speciation. Oxford (UK): Oxford University Press. [Google Scholar]
- Nosil P, Flaxman SM. 2011. Conditions for mutation-order speciation. Proc Biol Sci. 278:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P, Harmon LJ, Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends Ecol Evol. 24:145–156. [DOI] [PubMed] [Google Scholar]
- O’Grady P, DeSalle R. 2018. Hawaiian drosophila as an evolutionary model clade: days of future past. Bioessays. 40:e1700246. [DOI] [PubMed] [Google Scholar]
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo ZX, Meng J, et al. . 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 339:662–667. [DOI] [PubMed] [Google Scholar]
- Olson ME, Arroyo-Santos A. 2009. Thinking in continua: beyond the “adaptive radiation” metaphor. Bioessays. 31:1337–1346. [DOI] [PubMed] [Google Scholar]
- Orr HA 1995. The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics. 139:1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte D 1994. The Crickets of Hawaii: origin, systematics, and evolution. Philadelphia: Orthoptera Society/Academy of Natural Sciences of Philadelphia. [Google Scholar]
- Paccard A, Hanson D, Stuart Y, et al. . 2020. Repeatability of adaptive radiation depends on spatial scale: regional versus global replicates of stickleback in lake versus stream habitats. J Hered. 111:43–56. [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Pfennig KS. 2010. Character displacement and the origins of diversity. Am Nat. 176(Suppl 1):S26–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig DW, Pfennig KS. 2012a. Development and evolution of character displacement. Ann N Y Acad Sci. 1256:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig DW, Pfennig KS. 2012b. Evolution’s wedge: competition and the origins of diversity. Berkeley, CA: University of California Press. [Google Scholar]
- Phillips J, Linscott T, Rankin A, et al. . 2020. Archipelago-wide patterns of diversity and divergence among an endemic radiation of Galápagos land snails. J Hered. 111:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelstra JW, Richards EJ, Martin CH. 2018. Speciation in sympatry with ongoing secondary gene flow and a potential olfactory trigger in a radiation of Cameroon cichlids. Mol Ecol. 27:4270–4288. [DOI] [PubMed] [Google Scholar]
- Poff KE, Stever H, Reil JB, et al. . 2017. The native Hawaiian insect microbiome initiative: a critical perspective for Hawaiian insect evolution. Insects 8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TH, Cha DH, Linn CE Jr, Feder JL. 2012. On the scent of standing variation for speciation: behavioral evidence for native sympatric host races of Rhagoletis pomonella (Diptera: Tephritidae) in the southern United States. Evolution. 66:2739–2756. [DOI] [PubMed] [Google Scholar]
- Price PW 1980. Evolutionary biology of parasites. Princeton, New Jersey: Princeton University Press. [Google Scholar]
- Price TD 2010. The roles of time and ecology in the continental radiation of the Old World leaf warblers (Phylloscopus and Seicercus). Philos Trans R Soc Lond B Biol Sci. 365:1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JP, Clague DA. 2002. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proc Biol Sci. 269:2429–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. 2013. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat Commun. 4:1958. [DOI] [PubMed] [Google Scholar]
- Reddy S, Driskell A, Rabosky DL, Hackett SJ, Schulenberg TS. 2012. Diversification and the adaptive radiation of the vangas of Madagascar. Proc Biol Sci. 279:2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EJ, Martin CH. 2017. Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic adaptive radiation of trophic specialist pupfishes. PLoS Genet. 13:e1006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EJ, Poelstra JW, Martin CH. 2018. Don’t throw out the sympatric speciation with the crater lake water: fine-scale investigation of introgression provides equivocal support for causal role of secondary gene flow in one of the clearest examples of sympatric speciation. Evol Lett. 2:524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EJ, Servedio MR, Martin CH. 2019. Searching for sympatric speciation in the genomic era. Bioessays. 41:e1900047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Bermingham E. 2002a. The concept of the taxon cycle in biogeography. Global Ecol Biogeogr. 11:353–361. [Google Scholar]
- Ricklefs RE, Bermingham E. 2002b. The concept of the taxon cycle in biogeography. Global Ecol Biogeogr. 11:353–361. [Google Scholar]
- Roesti M, Gavrilets S, Hendry AP, Salzburger W, Berner D. 2014. The genomic signature of parallel adaptation from shared genetic variation. Mol Ecol. 23:3944–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum EB, Sarver BA, Brown JW, Des Roches S, Hardwick KM, Hether TD, Eastman JM, Pennell MW, Harmon LJ. 2012. Goldilocks meets Santa Rosalia: an ephemeral speciation model explains patterns of diversification across time scales. Evol Biol. 39:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser N, Kozak KM, Phillimore AB, Mallet J. 2015. Extensive range overlap between heliconiine sister species: evidence for sympatric speciation in butterflies? BMC Evol Biol. 15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueffler C, Van Dooren TJ, Leimar O, Abrams PA. 2006. Disruptive selection and then what? Trends Ecol Evol. 21:238–245. [DOI] [PubMed] [Google Scholar]
- Rubinoff D, Schmitz P. 2010. Multiple aquatic invasions by an endemic, terrestrial Hawaiian moth radiation. Proc Natl Acad Sci USA. 107:5903–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell RJ, Price TD. 2009. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol Evol. 24:394–399. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Nagel L, Wenrick Boughman J, Schluter D. 2000. Natural selection and parallel speciation in sympatric sticklebacks. Science. 287:306–308. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Nosil P. 2005. Ecological speciation. Ecol Lett. 8:336–352. [Google Scholar]
- Ryan PG, Bloomer P, Moloney CL, Grant TJ, Delport W. 2007. Ecological speciation in South Atlantic island finches. Science. 315:1420–1423. [DOI] [PubMed] [Google Scholar]
- Salzburger W 2018. Understanding explosive diversification through cichlid fish genomics. Nat Rev Genet. 19:705–717. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD Jr. 1999. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc Natl Acad Sci U S A. 96:11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D 2000. The ecology of adaptive radiation. Oxford (UK): Oxford University Press. [Google Scholar]
- Schluter D 2001. Ecology and the origin of species. Trends Ecol Evol. 16:372–380. [DOI] [PubMed] [Google Scholar]
- Schluter D 2009. Evidence for ecological speciation and its alternative. Science. 323:737–741. [DOI] [PubMed] [Google Scholar]
- Schluter D, McPhail JD. 1992. Ecological character displacement and speciation in sticklebacks. Am Nat. 140:85–108. [DOI] [PubMed] [Google Scholar]
- Seehausen O 2004. Hybridization and adaptive radiation. Trends Ecol Evol. 19:198–207. [DOI] [PubMed] [Google Scholar]
- Seehausen O 2006. Conservation: losing biodiversity by reverse speciation. Curr Biol. 16:R334–R337. [DOI] [PubMed] [Google Scholar]
- Seehausen O 2015. Process and pattern in cichlid radiations - inferences for understanding unusually high rates of evolutionary diversification. New Phytol. 207:304–312. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Butlin RK, Keller I, Wagner CE, Boughman JW, Hohenlohe PA, Peichel CL, Saetre GP, Bank C, Brännström A, et al. . 2014. Genomics and the origin of species. Nat Rev Genet. 15:176–192. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HD, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, et al. . 2008. Speciation through sensory drive in cichlid fish. Nature. 455:620–626. [DOI] [PubMed] [Google Scholar]
- Seehausen O, van Alphen JJM, Lande R. 1999. Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol Lett. 2:367–378. [Google Scholar]
- Seehausen O, Van Alphen JJ, Witte F. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811. [Google Scholar]
- Seehausen O, Wagner CE. 2014. Speciation in freshwater fishes. Annu Rev Ecol Evol Systematics. 45:621–651. [Google Scholar]
- Servedio MR, Noor MA. 2003. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol Systematics. 34:339–364. [Google Scholar]
- Shaw KL 2002. Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: what mtDNA reveals and conceals about modes of speciation in Hawaiian crickets. Proc Natl Acad Sci U S A. 99:16122–16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KL, Gillespie RG. 2016. Comparative phylogeography of oceanic archipelagos: Hotspots for inferences of evolutionary process. Proc Natl Acad Sci U S A. 113:7986–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG 1944. Tempo and mode in evolution. New York, N.Y: Columbia Univ. Press. [Google Scholar]
- Simpson GG 1953. The major features of evolution. New York, N.Y: Columbia Univ. Press. [Google Scholar]
- Stacy EA, Johansen JB, Sakishima T, Price DK, Pillon Y. 2014. Incipient radiation within the dominant Hawaiian tree Metrosideros polymorpha. Heredity (Edinb). 113:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy EA, Paritosh B, Johnson MA, Price DK. 2017. Incipient ecological speciation between successional varieties of a dominant tree involves intrinsic postzygotic isolating barriers. Ecol Evol. 7:2501–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy E, Sakishima T, Tharp H., Snow N. 2020. Isolation of Metrosideros (`Ohi`a) taxa on O`ahu increases with elevation and extreme environments. J Hered. 111:103–118. [DOI] [PubMed] [Google Scholar]
- Stacy EA, Sakishima T. (in review) Phylogeography of a highly dispersible landscape-dominant woody species complex. J Biogeogr. [Google Scholar]
- Stelkens RB, Schmid C, Selz O, Seehausen O. 2009. Phenotypic novelty in experimental hybrids is predicted by the genetic distance between species of cichlid fish. BMC Evol Biol. 9:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelkens RB, Young KA, Seehausen O. 2010. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution. 64:617–633. [DOI] [PubMed] [Google Scholar]
- Stervander M 2015. On speciation in birds – genomic signatures across space and time. PhD thesis, Lund University. [Google Scholar]
- Strecker U 2006. Genetic differentiation and reproductive isolation in a Cyprinodon fish species flock from Laguna Chichancanab, Mexico. Mol Phylogenet Evol. 39:865–872. [DOI] [PubMed] [Google Scholar]
- Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol Evol. 18:126–131. [Google Scholar]
- Stroud JT, Losos JB. 2016. Ecological opportunity and adaptive radiation. Annu Rev Ecol Evol Systematics. 47:507–532. [Google Scholar]
- Stroud JT, Losos JB. 2020. Bridging the process-pattern divide to understand the origins and early stages of adaptive radiation: a review of approaches with insights from studies of Anolis lizards. J Hered. 111:33–42. [DOI] [PubMed] [Google Scholar]
- Stuart YE, Campbell TS, Hohenlohe PA, Reynolds RG, Revell LJ, Losos JB. 2014. Rapid evolution of a native species following invasion by a congener. Science. 346:463–466. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Boughman JW, Groenenboom M, Sniatynski M, Schluter D, Gow JL. 2006. Speciation in reverse: morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Mol Ecol. 15:343–355. [DOI] [PubMed] [Google Scholar]
- Thibert‐Plante X, Gavrilets S. 2013. Evolution of mate choice and the so‐called magic traits in ecological speciation. Ecol Lett. 16:1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Snell-Rood EC. 2014. Ecology: diversity breeds complementarity. Nature. 515:44–45. [DOI] [PubMed] [Google Scholar]
- Vanoverbeke J, Urban MC, De Meester L. 2016. Community assembly is a race between immigration and adaptation: eco‐evolutionary interactions across spatial scales. Ecography 39:858–870. [Google Scholar]
- van Rijssel JC, Moser FN, Frei D, Seehausen O. 2018. Prevalence of disruptive selection predicts extent of species differentiation in Lake Victoria cichlids. Proc Roy Soc B: Biol Sci. 285:20172630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlanthen P, Bittner D, Hudson AG, Young KA, Müller R, Lundsgaard-Hansen B, Roy D, Di Piazza S, Largiader CR, Seehausen O. 2012. Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature. 482:357–362. [DOI] [PubMed] [Google Scholar]
- Wake DB 2006. Problems with species: patterns and processes of species formation in salamanders. Ann Missouri Botanical Garden. 93:8–24. [Google Scholar]
- Warren BH, Simberloff D, Ricklefs RE, Aguilée R, Condamine FL, Gravel D, Morlon H, Mouquet N, Rosindell J, Casquet J, et al. . 2015. Islands as model systems in ecology and evolution: prospects fifty years after MacArthur-Wilson. Ecol Lett. 18:200–217. [DOI] [PubMed] [Google Scholar]
- Weber MG, Wagner CE, Best RJ, Harmon LJ, Matthews B. 2017. Evolution in a community context: on integrating ecological interactions and macroevolution. Trends Ecol Evol. 32:291–304. [DOI] [PubMed] [Google Scholar]
- Wellborn GA, Langerhans RB. 2015. Ecological opportunity and the adaptive diversification of lineages. Ecol Evol. 5:176–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ 2003. Developmental plasticity and evolution. Oxford: Oxford University Press. [Google Scholar]
- Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T. 2007. The genetic basis of a plant-insect coevolutionary key innovation. Proc Natl Acad Sci U S A. 104:20427–20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat CW, Watt WB, Pollock DD, Schulte PM. 2006. From DNA to fitness differences: sequences and structures of adaptive variants of Colias phosphoglucose isomerase (PGI). Mol Biol Evol. 23:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall JB, Hodges SA. 2007. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 447:706–709. [DOI] [PubMed] [Google Scholar]
- Wilson EO 1961. The nature of the taxon cycle in the Melanesian ant fauna. Am Nat. 95:169–193. [Google Scholar]
- Wogan G, Wang I. 2019. Environmental niche disparity during the adaptive radiations of the greater antillean Anolis lizards. J Hered. [Google Scholar]
- Wood HM, Matzke NJ, Gillespie RG, Griswold CE. 2013. Treating fossils as terminal taxa in divergence time estimation reveals ancient vicariance patterns in the palpimanoid spiders. Syst Biol. 62:264–284. [DOI] [PubMed] [Google Scholar]
- Xu M, Shaw KL. 2019. The genetics of mating song evolution underlying rapid speciation: linking quantitative variation to candidate genes for behavioral isolation. Genetics. 211:1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Shaw KL. 2020. Spatial mixing between calling males of two closely related, sympatric crickets suggests beneficial heterospecific interactions in a non-adaptive radiation. J Hered. 111:84–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.