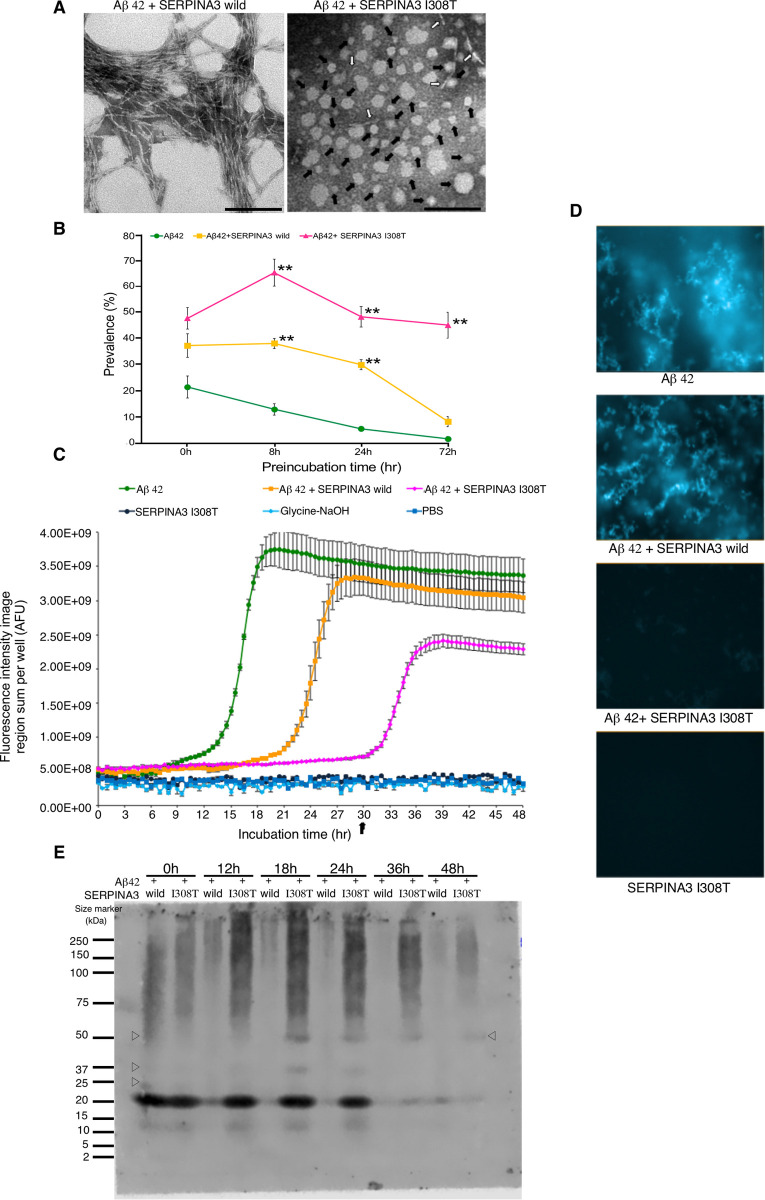
Fig 3. Regulation of various Aβ peptide forms by human polymorphic SERPINA3.
(A) Representative TEM images of Aβ peptide at 72 hr preincubation. Typical fibrillar form was observed in Aβ 42 + SERPINA3 wild type; whereas Aβ 42 + SERPINA3 I308T still showed irregularly shaped oligomers as indicated by solid arrow and while open arrow indicated protofibrillar form. Scale bar: 100 nm. (B) Kinetics of amyloid β peptide oligomerization in presence of human SERPINA3. Here, x-axis shows prevalence of oligomer rich observation fields (in percentage) and y-axis shows preincubation time (hr). Significance levels were tested Aβ 42 + SERPINA3 I308T vs. Aβ 42 + SERPINA3 wild; Aβ 42 + SERPINA3 I308T vs. Aβ 42; Aβ 42 + SERPINA3 wild vs. Aβ 42. (n = 3, ** P ≤ 0.01. One-way ANOVA with Tukey and Kramer’s honestly significance difference test was used). (C) Thioflavin T fluorescence assay (data represent Mean ± SEM, n = 3). AFU refers arbitrary fluorescence units. (D) Representative images of thioflavin T fluorescence assay at 30 hrs. (E) Representative image of gradient gel native PAGE, showing Aβ 42 peptide in presence of human SERPINA3 recombinant proteins, at 0 to 48 hrs of preincubation. Open triangle showed presence of oligomeric Aβ peptide conformations. See also in S3 Fig.