Abstract
Fruits and vegetables contain many bioactive components that may contribute to improved survival after diagnosis of breast cancer, however, evidence to date is insufficient. We prospectively assessed the associations of post-diagnostic fruit and vegetable consumption with breast cancer-specific and all-cause mortality among 8,927 women with stage I–III breast cancer identified during follow-up of the Nurses’ Health Study (NHS; 1980–2010) and NHSII (1991–2011), using a validated food frequency questionnaire completed every 4 years after diagnosis. We prospectively documented 2,521 deaths, including 1,070 from breast cancer through follow-up until 2014 in the NHS and 2015 in the NHSII. Total fruit and vegetable and total vegetable consumption was related to lower all-cause [HRQ5vsQ1, 0.82; 95% confidence interval (CI), 0.71–0.94; Ptrend = 0.004, and HRQ5vsQ1, 0.84; 95% CI, 0.72–0.97; Ptrend = 0.001, respectively], but not breast cancer-specific mortality. Total fruit consumption was not related to breast cancer-specific or all-cause mortality. Greater intake of green leafy and cruciferous vegetables was associated with lower all-cause mortality. Each 2 servings/week of blueberries was associated with a 25% (HR, 0.75; 95% CI, 0.60–0.94) lower breast cancer-specific and a 17% (HR, 0.83; 95% CI, 0.72–0.96) lower all-cause mortality. In contrast, higher fruit juice consumption was associated with higher breast cancer-specific (HRQ5vsQ1, 1.33; 95% CI, 1.09–1.63; Ptrend = 0.002) and all-cause mortality (HRQ5vsQ1, 1.19; 95% CI, 1.04–1.36; Ptrend = 0.003). Apple juice largely accounted for these higher risks and orange juice was not associated with risk. Higher post-diagnostic fruit and vegetable consumption among breast cancer survivors was not associated with breast cancer-specific mortality. However, our findings suggest that higher vegetable consumption, particularly green leafy and cruciferous vegetables, was associated with better overall survival among patients with breast cancer. Higher fruit juice consumption, but not orange juice, was associated with poorer breast cancer-specific and all-cause survival.
Significance: A large-scale study shows that high fruit and vegetable consumption may be associated with better overall survival among breast cancer patients, while high fruit juice consumption may be associated with poorer prognosis.
Introduction
The population of breast cancer survivors has increased over time, and identification of ways to minimize disease progression has become increasingly important. Survival rates after breast cancer diagnosis differ widely, raising the possibility that improvements in lifestyle as part of breast cancer care may increase life expectancy. However, survival benefits associated with dietary factors remain largely unclear (1–13).
Fruits and vegetables consist of many potentially anti-carcinogenic substances (14, 15) which may play an important role in decreasing mortality. However, the World Cancer Research Fund/American Institute for Cancer Research concluded that data were insufficient to recommend high post-diagnostic fruit and vegetable consumption for breast cancer survival (16). In a recent meta-analysis, pre- or post-diagnostic fruit or vegetable consumption was not significantly associated with overall mortality in patients with breast cancer (17). Furthermore, fruits and vegetables differ greatly in composition and examination of specific subgroups may provide more relevant information for survival. For example, some evidence suggests that cruciferous vegetables may affect breast cancer survival (9), but overall, the evidence is sparse and results inconsistent (12).
Using combined data from the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHSII), we evaluated the relationship between post-diagnostic fruit and vegetable consumption and breast cancer survival, using repeated assessments and accounting for pre-diagnostic diet. As the associations may vary by hormone receptor status and disease stage, we conducted analyses separately by these variables.
Materials and Methods
Study Population
As ongoing cohort studies, the NHS began in 1976 with an enrollment of 121,700 US female nurses aged 30–55 years, and the NHSII started in 1989 with an enrollment of 116,429 female nurses aged 25–42 years. For this study, we included women with invasive breast cancer that diagnosed from 1980 to 2010 in the NHS, and from 1991 to 2011 in the NHSII. After excluding women with prior cancer diagnosis (except non-melanoma skin cancer) or death before the baseline food frequency questionnaire (FFQ) (1980 in the NHS and 1991 in the NHSII), implausible total energy intake (<600 or >3500 kcal/day), left blank more than 70 food items, left blank all fruit and/or vegetable items, missing diet information at least 12 months after diagnosis, a cancer diagnosis (except nonmelanoma skin cancer) before breast cancer, stage IV disease at diagnosis, or missing information on disease stage, 8,927 patients with breast cancer were included in the analyses.
Completion of the questionnaire was considered to imply informed consent when the study protocol was approved in 1976 (NHS) and 1989 (NHSII) by the Institutional Review Boards of the Brigham and Women’s Hospital (Boston, MA) and Harvard T.H. Chan School of Public Health (Boston, MA), and those of participating registries as required. The studies were conducted in accordance with recognized ethical guidelines (Declaration of Helsinki).
Assessment of Dietary Intake
In the NHS, a 61-item semi-quantitative FFQ was first administered in 1980. Subsequently, the FFQ was expanded to 116–130 items in 1984, 1986, and every four years thereafter. In the NHSII, a ~130-item FFQ was administered in 1991 and every four years thereafter (questionnaires available at http://www.nurseshealthstudy.org/participants/questionnaires). Post-diagnostic fruit, vegetable, and fruit juice consumption was collected from FFQs completed at least 12 months after diagnosis. To reduce measurement error and within-person variation and evaluate dietary intake over a long period after diagnosis, we calculated the cumulative average of postdiagnostic fruit, vegetable, and fruit juice intake from all available FFQs after diagnosis up to the start of each 2-year follow-up interval. The FFQ has been extensively validated in our cohorts using multiple analyses by comparison with more detailed methods (18–20) and biomarkers of intake (19). For example, for the validity of the questionnaire, long-term intake was compared with weighed diet records among NHS participants: the mean of correlation coefficients was 0.80 for apples and 0.69 for broccoli, calculated using the 1986 FFQ and multiple dietary records obtained in 1986 (18). Fruit and vegetable consumption calculated using these FFQs has also predicted a lower risk of type 2 diabetes (21) and coronary heart disease (22).
Individual items reported in the FFQs were summed to create total fruit (not including juices), total vegetable (not including potatoes), and total fruit juice intake (Table S1). Vegetable subgroups included leafy greens, yellow/orange, tomatoes, cruciferous, and other. We also grouped fruits and vegetables based on amount of specific nutrients including vitamin C (≥40mg/100g), α-carotene (≥3000mcg/100g), β-carotene (≥3000mcg/100g), and lutein (≥10mg/100g) (22–25).
Ascertainment of Breast Cancer and Death
Breast cancers were identified through self-report on the biennial questionnaires. We then requested permission from participants (or next of kin) to access medical records and pathology reports to confirm the diagnosis and obtain information on tumor characteristics, disease stage, estrogen receptor (ER) and progesterone receptor (PR) status, and other relevant information. Deaths were reported by family members or the postal service or determined through search of the National Death Index. The cause of death was ascertained by physician review of the death certificate and medical record.
Covariates
Data on post-diagnostic body mass index (BMI), smoking status, physical activity, and aspirin use were collected from biennial questionnaires. To minimize assessment during active treatment, only measurements taken at least 12 months after diagnosis were considered. To reduce the possibility of reverse causation, we calculated the cumulative averages of post-diagnostic BMI and physical activity using 4-year lagged data. We calculated pre-diagnostic BMI from the last questionnaire returned before diagnosis. We also considered changes in BMI from pre- to post-diagnosis, age at menopause, menopausal status, postmenopausal hormone use, and oral contraceptive use before diagnosis. In addition, we collected information about breast cancer characteristics, including age at diagnosis, disease stage, ER/PR status, self-reported radiotherapy, chemotherapy, and hormonal treatment.
Statistical analysis
We calculated person-time of follow-up from the return date of the FFQ that was used for the first post-diagnostic assessment to the end of the study period (June 1, 2014 in the NHS and June 1, 2015 in the NHSII) or death, whichever came first. We evaluated breast cancer-specific, all-cause, and cardiovascular disease (CVD) mortality as endpoints.
In the combined NHS and NSHII data, Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Participants diagnosed with breast cancer were divided into quintiles according to the cumulative average of dietary intake. For vegetables high in lutein, participants were divided into quartiles according to the cumulative average of intake. For apple juice, participants who did not consume it were assigned to the lowest group and others were divided into 3 categories, as was other juices.
The median value for each category was used for tests for trend, modeled as a continuous variable. Models were stratified by cohort and adjusted for age at diagnosis and calendar year of diagnosis. In the analysis of breast cancer-specific mortality, follow-up was censored with death from other causes. Multivariable models included time between diagnosis and first FFQ after diagnosis, calendar year at start of follow-up of each-2-year questionnaire cycle, potential predictors of breast cancer survival and total energy intake (see Table 2 footnote). For missing covariates, we used the carried-forward method for continuous variables and missing indicator method for categorical variables. To address role of other dietary factors, we also evaluated the associations after additionally controlling for the post-diagnostic modified alternate healthy eating index (AHEI), glycemic load (GL), total fat, and sugar-sweetened beverage (SSB) intake. We adjusted for cereal fiber, not total fiber, because it is redundant variable. Furthermore, in secondary analyses, the association of post-diagnostic fruit and vegetable consumption was examined by additionally adjusting for pre-diagnostic fruit and vegetable consumption, which was calculated from the last FFQ reported before breast cancer diagnosis. We also evaluated the associations with changes in fruit and vegetable intake from pre- to post-diagnosis, controlling for pre-diagnostic intake. We also evaluated the risk of breast cancer-specific and all-cause mortality with cross-classification of pre- and post-diagnostic intake (high/high, low/high, high/low, compared with low/low). Less than 3 servings/day of total fruit and vegetable intake was considered low intake, as was less than 2 servings/day for total fruit and for total vegetable intake.
We conducted stratified analyses to evaluate potential effect modifiers of the fruit and vegetable association with survival by lifestyle and clinical-pathological factors including age at diagnosis (<60 vs. ≥60 years), post-diagnostic smoking status (never vs. ever), alcohol consumption (<3.5 vs. ≥3.5 g/day), BMI (<25 vs. ≥25 kg/m2), physical activity (<10 vs. ≥10 MET-hrs/week), aspirin use (never vs. ever), ER status (ER+ vs. ER-), and disease stage (I, II, III). In stratified analyses, the HR of mortality was calculated for two servings/day increments of total fruit and vegetable, total fruit, and total vegetable intake. The p value for interaction was calculated using the Wald test. To assess heterogeneity by causes of death, we compared the HR between competing causes of death, using data duplication methods (26). We also performed 4-year time-lagged analysis to examine potential bias due to reverse causation. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC) with a two-sided p-value of <0.05.
Results
Baseline characteristics of participants at diagnosis
During following up of the 8,927 eligible women diagnosed with stage I–III breast cancer, we documented 2,521 deaths, of which 1,070 were classified as breast cancer-specific from the date of return of the first post-diagnostic FFQ to end of the study period (mean follow-up time = 11.5 years, up to 30 years). Participants with higher total fruit and vegetable intake tended to be more physically active and were more likely to use postmenopausal hormone. They were also less likely to smoke or drink alcohol, and they consumed more dietary fiber and less total fat and animal fat (Table 1). Stage of breast cancer did not differ across quintiles of total fruit and vegetable consumption.
Table 1.
Characteristics of 8,927 women with breast cancer in the NHS and NHSII reported after diagnosis, according to quintile of post-diagnostic total fruit and vegetable intake.
| Total fruits and vegetables |
|||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Number | 1,922 | 1,791 | 1,741 | 1,698 | 1,775 |
| Mean | |||||
| Total fruit intake, servings/day | 0.6 | 1.1 | 1.5 | 1.9 | 2.9 |
| Total vegetable intake, servings/day | 1.4 | 2.2 | 2.9 | 3.7 | 5.6 |
| Age at diagnosis, years | 59.3 | 58.9 | 58.7 | 58.2 | 58.1 |
| BMI, kg/m2 | 26.4 | 26.9 | 26.4 | 26.7 | 26.4 |
| Physical activity, MET-hrs/week | 12.0 | 15.7 | 18.0 | 19.8 | 23.9 |
| Alcohol consumption, g/day | 6.0 | 5.9 | 5.7 | 5.7 | 5.2 |
| Total fiber intake, g/day | 15.5 | 18.6 | 20.3 | 22.1 | 26.0 |
| Animal fat intake, % energy/day | 15.8 | 15.1 | 14.6 | 13.8 | 12.5 |
| Total fat intake, % energy/day | 32.2 | 31.0 | 30.8 | 30.0 | 28.9 |
| Total energy intake, kcal/day | 1,376 | 1,552 | 1,700 | 1,871 | 2,134 |
| % | |||||
| Current smokers | 15 | 10 | 9 | 6 | 5 |
| Ever used oral contraceptives | 59 | 57 | 58 | 56 | 57 |
| Ever used postmenopausal hormone | 47 | 48 | 48 | 48 | 49 |
| Current use of aspirin | 43 | 46 | 43 | 46 | 44 |
| Pre-menopausal at diagnosis | 25 | 27 | 26 | 26 | 27 |
| Stage of breast cancer | |||||
| I | 60 | 60 | 61 | 60 | 59 |
| II | 30 | 31 | 29 | 30 | 30 |
| III | 10 | 9 | 10 | 10 | 11 |
| Estrogen receptor status | |||||
| Positive | 76 | 77 | 77 | 75 | 78 |
| Negative | 18 | 16 | 18 | 18 | 15 |
| Missing | 6 | 7 | 5 | 7 | 7 |
| Treatment | |||||
| Radiotherapy | 56 | 57 | 58 | 55 | 57 |
| Chemotherapy | 45 | 45 | 46 | 46 | 47 |
| Hormonal treatment | 69 | 69 | 69 | 68 | 71 |
Post-diagnostic total fruit and vegetable consumption was associated with lower all-cause mortality (HRQ5vsQ1=0.82, 95%CI=0.71–0.94; Ptrend=0.004), but not breast cancer-specific mortality (HRQ5vsQ1=0.88, 95%CI=0.71–1.09; Ptrend=0.55) (Table 2). The associations between total fruit and vegetable consumption and all-cause mortality remained significant after additional adjustment for pre-diagnostic total fruit and vegetable consumption or post-diagnostic dietary GL or total fat intake. However, there was no significant association between total fruit and vegetable intake and all-cause mortality after additional adjustment for modified AHEI (excluding fruit, vegetable, and alcohol scores) (HRQ5vsQ1=0.88, 95%CI=0.75–1.02; Ptrend=0.07). Higher post-diagnostic total vegetable consumption was associated with lower all-cause (HRQ5vsQ1=0.84, 95%CI=0.72–0.97; Ptrend=0.001), but not breast cancer-specific mortality. The associations between total vegetable consumption and all-cause mortality remained significant after additional adjustment for post-diagnostic GL, total fat, or cereal fiber intake. Additional adjustment for pre-diagnostic vegetable intake resulted in a stronger inverse association between consumption of total vegetables and all-cause mortality. The association was attenuated after additional adjustment for modified AHEI (excluding fruit, vegetable, and alcohol scores) (HRQ5vsQ1=0.89, 95%CI=0.76–1.02; Ptrend=0.02). Fruit consumption was not associated with breast cancer-specific or all-cause mortality.
Table 2:
Post-diagnostic fruit and vegetable consumption in relation to mortality after breast cancer diagnosis (n=8,927 women) in the NHS and NHSII.
| Consumption Levels |
Ptrend | Per 2 servings/day | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Total fruits and vegetables | |||||||
| Median intake, servings/day | 2.2 | 3.4 | 4.3 | 5.5 | 7.4 | ||
| Breast cancer specific mortality | |||||||
| Number of deaths | 240 | 190 | 207 | 221 | 212 | ||
| Model 1 | 1 | 0.74 (0.61–0.90) | 0.80 (0.66–0.96) | 0.85 (0.71–1.02) | 0.84 (0.69–1.00) | 0.32 | 0.97 (0.90–1.03) |
| Model 2 | 1 | 0.79 (0.65–0.96) | 0.91 (0.75–1.11) | 0.90 (0.74–1.10) | 0.88 (0.71–1.09) | 0.55 | 0.98 (0.90–1.06) |
| All-cause mortality | |||||||
| Number of deaths | 586 | 542 | 475 | 473 | 445 | ||
| Model 1 | 1 | 0.88 (0.78–0.99) | 0.78 (0.69–0.88) | 0.78 (0.69–0.88) | 0.74 (0.65–0.84) | <0.0001 | 0.89 (0.85–0.93) |
| Model 2 | 1 | 0.91 (0.81–1.02) | 0.89 (0.79–1.02) | 0.83 (0.73–0.95) | 0.82 (0.71–0.94) | 0.004 | 0.93 (0.88–0.98) |
| Total fruits | |||||||
| Median intake, servings/day | 0.5 | 1.0 | 1.4 | 1.9 | 2.8 | ||
| Breast cancer-specific mortality | |||||||
| Number of deaths | 218 | 201 | 214 | 203 | 234 | ||
| Model 1 | 1 | 0.87 (0.72–1.06) | 0.90 (0.74–1.09) | 0.85 (0.70–1.03) | 0.98 (0.81–1.18) | 0.97 | 1.00 (0.86–1.17) |
| Model 2 | 1 | 1.06 (0.87–1.29) | 1.10 (0.90–1.34) | 1.09 (0.88–1.33) | 1.03 (0.83–1.26) | 0.93 | 1.01 (0.85–1.19) |
| All-cause mortality | |||||||
| Number of deaths | 512 | 495 | 498 | 480 | 536 | ||
| Model 1 | 1 | 0.89 (0.79–1.01) | 0.84 (0.75–0.96) | 0.79 (0.70–0.89) | 0.85 (0.75–0.96) | 0.008 | 0.87 (0.79–0.96) |
| Model 2 | 1 | 1.05 (0.92–1.19) | 0.98 (0.86–1.11) | 0.98 (0.85–1.11) | 0.93 (0.81–1.07) | 0.18 | 0.93 (0.83–1.03) |
| Total vegetables | |||||||
| Median intake, servings/day | 1.4 | 2.2 | 2.9 | 3.6 | 5.1 | ||
| Breast cancer-specific mortality | |||||||
| Number of deaths | 227 | 213 | 218 | 217 | 195 | ||
| Model 1 | 1 | 0.90 (0.74–1.08) | 0.91 (0.75–1.09) | 0.90 (0.75–1.09) | 0.84 (0.69–1.02) | 0.11 | 0.93 (0.84–1.02) |
| Model 2 | 1 | 0.92 (0.76–1.11) | 0.94 (0.77–1.15) | 0.92 (0.75–1.12) | 0.87 (0.70–1.08) | 0.25 | 0.94 (0.84–1.05) |
| All-cause mortality | |||||||
| Number of deaths | 600 | 564 | 505 | 459 | 393 | ||
| Model 1 | 1 | 0.94 (0.84–1.05) | 0.87 (0.77–0.98) | 0.80 (0.71–0.91) | 0.72 (0.64–0.82) | <0.0001 | 0.84 (0.78–0.89) |
| Model 2 | 1 | 1.00 (0.89–1.12) | 0.96 (0.85–1.09) | 0.82 (0.72–0.94) | 0.84 (0.72–0.97) | 0.001 | 0.89 (0.82–0.95) |
Model 1 stratified by cohort and adjusted for age at diagnosis (year) and calendar year of diagnosis.
Model 2 stratified by cohort and adjusted for age at diagnosis (year), calendar year of diagnosis, time between diagnosis and first FFQ (year), calendar year at start of follow-up of each-2-year questionnaire cycle, pre-diagnostic BMI (<20, 20 to <22.5, 22.5 to <25, 25.0 to <30, 30 to <35, ≥35 kg/m2, missing), BMI change after diagnosis (no change (≥−0.5 to ≤0.5 kg/m2), decrease (<−0.5 kg/m2), increase (>0.5–2 kg/m2), increase (>2 kg/m2), missing), post-diagnostic smoking (never, past, current 1–14/day, current 15–24/day, current ≥25/day, missing), post-diagnostic physical activity (<5, 5 to <11.5, 11.5 to <22, ≥22 MET-h/week, missing), oral contraceptive use (ever, never), post-diagnostic alcohol consumption (<0.15, 0.15 to <2.0, 2.0 to 7.5, ≥7.5 g/day), post-diagnostic total energy intake (quintiles, kcal/day), pre-diagnostic menopausal status, age at menopause, and postmenopausal hormone use (premenopausal, postmenopausal and age at menopause<50 year and never postmenopausal hormone use, postmenopausal and age at menopause<50 year and past postmenopausal hormone use, postmenopausal and age at menopause<50 year and current postmenopausal hormone use, postmenopausal and age at menopause≥50 year and never postmenopausal hormone use, postmenopausal and age at menopause≥50 year and past postmenopausal hormone use, postmenopausal and age at menopause≥50 year and current postmenopausal hormone use, missing), post-diagnostic aspirin use (never, past, current, missing), race (white, other), stage of disease (I, II, III), ER/PR status (ER/PR positive, ER positive and PR negative, ER/PR negative, missing), radiotherapy (yes, no, missing), chemotherapy (yes, no, missing), and hormonal treatment (yes, no, missing).
High post-diagnostic fruit juice intake was associated with both higher breast cancer-specific (HRQ5vsQ1=1.33, 95%CI=1.09–1.63; Ptrend=0.002) and all-cause (HRQ5vsQ1=1.19, 95%CI=1.04–1.36; Ptrend=0.003) mortality (Table 3). Although we did not observe a significant association for breast cancer-specific and all-cause mortality in the Model 1, none of the covariates accounted individually for the difference in HRs for fruit juice consumption between the age-adjusted and the multivariable models. These associations with breast cancer-specific mortality were significant after additionally adjusting for total fiber intake, dietary GL or modified AHEI (excluding SSB and alcohol scores). However, the associations of fruit juice with all-cause mortality were attenuated after adjusting for dietary GL (HRQ5vsQ1=1.13, 95%CI=0.99–1.30; Ptrend=0.05). Additional adjustment for pre-diagnostic fruit juice intake resulted in a stronger positive association between consumption of fruit juice and breast cancer-specific and all-cause mortality. Furthermore, the associations between post-diagnostic fruit juice consumption and breast cancer-specific and all-cause mortality remained significant after additional adjustment for post-diagnostic SSB: HRQ5vsQ1=1.27, 95%CI=1.04–1.55; Ptrend=0.008 for breast cancer-specific mortality and HRQ5vsQ1=1.16; 95% CI, 1.02– 1.32; Ptrend = 0.01 for all-cause mortality. Higher risk of breast cancer-specific and all-cause mortality was observed with apple juice and with other juices (not including apple and orange juices). Orange juice was not associated with higher risk of breast cancer-specific or all-cause mortality (Table 3).
Table 3:
Post-diagnostic fruit juice consumption in relation to mortality after breast cancer diagnosis in NHS and NHSII.
| Consumption Levels |
Ptrend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Fruit juices | ||||||
| Median intake, servings/day | 0 | 0.3 | 0.8 | 1.5 | 2.1 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 199 | 175 | 215 | 225 | 250 | |
| Model 1 | 1 | 0.78 (0.64–0.96) | 0.94 (0.77–1.14) | 0.98 (0.81–1.19) | 1.06 (0.88–1.28) | 0.05 |
| Model 2 | 1 | 0.97 (0.79–1.19) | 1.18 (0.97–1.44) | 1.11 (0.91–1.36) | 1.33 (1.09–1.63) | 0.002 |
| All-cause mortality | ||||||
| Number of deaths | 451 | 432 | 501 | 550 | 579 | |
| Model 1 | 1 | 0.91 (0.80–1.04) | 0.98 (0.86–1.11) | 0.97 (0.86–1.10) | 1.03 (0.91–1.17) | 0.26 |
| Model 2 | 1 | 0.98 (0.86–1.13) | 1.06 (0.93–1.20) | 1.05 (0.93–1.20) | 1.19 (1.04–1.36) | 0.003 |
| Orange juice | ||||||
| Median intake, servings/day | 0 | 0.1 | 0.4 | 1.1 | 1.5 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 196 | 183 | 251 | 194 | 232 | |
| Model 1 | 1 | 0.90 (0.74–1.11) | 0.95 (0.79–1.15) | 0.88 (0.72–1.07) | 0.98 (0.81–1.19) | 0.95 |
| Model 2 | 1 | 1.11 (0.90–1.37) | 1.07 (0.88–1.29) | 1.25 (1.01–1.54) | 1.09 (0.89–1.33) | 0.31 |
| All-cause mortality | ||||||
| Number of deaths | 470 | 417 | 559 | 502 | 546 | |
| Model 1 | 1 | 0.95 (0.83–1.08) | 0.95 (0.84–1.07) | 0.96 (0.85–1.09) | 0.91 (0.80–1.03) | 0.25 |
| Model 2 | 1 | 0.99 (0.87–1.14) | 0.96 (0.85–1.10) | 1.04 (0.91–1.19) | 0.98 (0.86–1.11) | 0.92 |
| Apple juice | ||||||
| Median intake, servings/day | 0 | 0.04 | 0.1 | 0.3 | ||
| Breast cancer-specific mortality | ||||||
| Number of deaths | 612 | 90 | 156 | 182 | ||
| Model 1 | 1 | 0.58 (0.46–0.73) | 1.14 (0.95–1.35) | 1.26 (1.07–1.49) | 0.0005 | |
| Model 2 | 1 | 0.86 (0.68–1.09) | 1.14 (0.95–1.36) | 1.32 (1.11–1.57) | 0.0007 | |
| All-cause mortality | ||||||
| Number of deaths | 1,481 | 308 | 295 | 387 | ||
| Model 1 | 1 | 0.97 (0.86–1.10) | 1.00 (0.88–1.13) | 1.24 (1.11–1.39) | 0.0003 | |
| Model 2 | 1 | 0.98 (0.86–1.11) | 1.03 (0.90–1.17) | 1.24 (1.11–1.40) | 0.0003 | |
| Other juices | ||||||
| Median intake, servings/day | 0 | 0.1 | 0.2 | 0.7 | ||
| Breast cancer-specific mortality | ||||||
| Number of deaths | 368 | 214 | 206 | 257 | ||
| Model 1 | 1 | 0.89 (0.75–1.05) | 0.95 (0.80–1.12) | 1.14 (0.97–1.34) | 0.03 | |
| Model 2 | 1 | 0.98 (0.82–1.16) | 1.18 (0.99–1.41) | 1.31 (1.11–1.55) | 0.0004 | |
| All-cause mortality | ||||||
| Number of deaths | 840 | 537 | 504 | 602 | ||
| Model 1 | 1 | 1.05 (0.94–1.17) | 1.09 (0.98–1.22) | 1.18 (1.06–1.31) | 0.002 | |
| Model 2 | 1 | 1.05 (0.94–1.17) | 1.15 (1.02–1.29) | 1.25 (1.12–1.39) | <0.0001 | |
See table 2 footnote
We also examined the change in fruit and vegetable consumption from before to after diagnosis. One or more servings/day decrease in total fruit and vegetable intake from pre- to post-diagnosis was associated with a higher all-cause mortality (HR=1.14, 95%CI=1.01–1.27) (Figure S1–B). A ≥1 servings/day decrease in total vegetable intake was also associated with a higher risk of all-cause mortality (HR=1.16, 95%CI=1.02–1.30) (Figure S1–F). A decrease in post-diagnostic total fruit intake was not significantly associated with increased mortality among breast cancer survivors. Furthermore, we evaluated the risk of breast cancer-specific and all-cause mortality with cross-classification of pre- and post-diagnostic intake (high/high, low/high, high/low, compared with low/low). Compared to both low pre- and low post-diagnostic vegetable consumption, we did not observe significant decreased or increased risk of breast cancer-specific mortality among women with low pre- (<2 servings/day) and high post-diagnostic (≥2 servings/day), both high pre- and post-diagnostic, or high pre- and low post-diagnostic intake (Figure S2E). Risk of all-cause mortality was higher among women who had high pre- (≥2 servings/day) and low post-diagnostic (<2 servings/day) vegetable consumption (HR=1.22, 95%CI=1.05–1.42), however, there was no significant decreased or increased risk of all-cause mortality among women with both high pre- and post-diagnostic vegetable intake (HR=1.03; 95%CI=0.91–1.16) or low pre- and high post-diagnostic intake (HR=0.96; 95%CI=0.83–1.11) (Figure S2–F). No significant associations were observed for total fruit and vegetable or total fruit intake (Supplementary Figure S2A, S2B, S2C, and S2D).
Among subgroup of vegetables, post-diagnostic green leafy vegetable intake was associated with lower risk of all-cause mortality (HRQ5vsQ1=0.80, 95%CI=0.70–0.91; Ptrend<0.0001), but not breast cancer-specific mortality (Table 4). Intake of cruciferous vegetables was associated with lower risk of all-cause mortality (HRQ5vsQ1=0.87, 95%CI=0.76–0.99; Ptrend=0.01). Also, lower all-cause mortality was observed with greater intake of post-diagnostic fruits and vegetables high in vitamin C (HRQ5vsQ1=0.86, 95%CI=0.75–0.98; Ptrend=0.02) as well as vegetables high in β-carotene with all-cause mortality (HRQ5vsQ1=0.80, 95%CI=0.70–0.91; Ptrend<0.0001) (Table S2). These associations remained significant after additional adjustment for cereal fiber intake. Additional adjustment for pre-diagnostic intake of these vegetables did not appreciably change the results. Furthermore, green leafy vegetables and vegetables high in β-carotene were somewhat associated with lower CVD mortality (Table S3).
Table 4:
Post-diagnostic subgroups of vegetable consumption in relation to mortality after breast cancer diagnosis in the NHS and NHSII.
| Consumption Levels |
Ptrend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Green leafy vegetables | ||||||
| Median intake, servings/day | 0.2 | 0.5 | 0.8 | 1.1 | 1.7 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 258 | 236 | 198 | 196 | 180 | |
| Model 1 | 1 | 0.85 (0.71–1.01) | 0.74 (0.61–0.89) | 0.73 (0.61–0.88) | 0.75 (0.62–0.91) | 0.002 |
| Model 2 | 1 | 1.04 (0.86–1.24) | 0.91 (0.75–1.11) | 1.00 (0.82–1.21) | 0.90 (0.74–1.10) | 0.26 |
| All-cause mortality | ||||||
| Number of deaths | 660 | 537 | 501 | 433 | 388 | |
| Model 1 | 1 | 0.82 (0.73–0.92) | 0.76 (0.68–0.86) | 0.67 (0.60–0.76) | 0.67 (0.59–0.76) | <0.0001 |
| Model 2 | 1 | 0.97 (0.86–1.09) | 0.87 (0.77–0.99) | 0.82 (0.72–0.93) | 0.80 (0.70–0.91) | <0.0001 |
| Yellow/orange vegetables | ||||||
| Median intake, servings/day | 0.1 | 0.2 | 0.3 | 0.4 | 0.7 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 215 | 234 | 219 | 190 | 212 | |
| Model 1 | 1 | 0.99 (0.82–1.19) | 0.87 (0.72–1.05) | 0.83 (0.68–1.01) | 0.89 (0.73–1.07) | 0.12 |
| Model 2 | 1 | 1.03 (0.85–1.24) | 1.10 (0.90–1.33) | 1.07 (0.87–1.31) | 1.08 (0.88–1.32) | 0.52 |
| All-cause mortality | ||||||
| Number of deaths | 543 | 545 | 514 | 440 | 479 | |
| Model 1 | 1 | 0.92 (0.82–1.04) | 0.84 (0.74–0.94) | 0.79 (0.69–0.89) | 0.79 (0.70–0.89) | <0.0001 |
| Model 2 | 1 | 0.98 (0.87–1.11) | 0.95 (0.84–1.08) | 0.93 (0.81–1.06) | 0.95 (0.83–1.09) | 0.42 |
| Tomatoes | ||||||
| Median intake, servings/day | 0.2 | 0.3 | 0.5 | 0.7 | 1.1 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 212 | 203 | 199 | 214 | 242 | |
| Model 1 | 1 | 0.93 (0.77–1.13) | 0.85 (0.70–1.04) | 0.90 (0.74–1.09) | 1.04 (0.86–1.25) | 0.42 |
| Model 2 | 1 | 1.01 (0.83–1.24) | 1.00 (0.81–1.22) | 1.04 (0.85–1.28) | 0.96 (0.78–1.17) | 0.65 |
| All-cause mortality | ||||||
| Number of deaths | 580 | 500 | 492 | 491 | 455 | |
| Model 1 | 1 | 0.92 (0.82–1.04) | 0.88 (0.78–0.99) | 0.90 (0.80–1.02) | 0.87 (0.77–0.99) | 0.06 |
| Model 2 | 1 | 0.93 (0.82–1.06) | 0.94 (0.83–1.07) | 1.01 (0.89–1.15) | 0.93 (0.81–1.06) | 0.60 |
| Cruciferous vegetables | ||||||
| Median intake, servings/day | 0.1 | 0.2 | 0.4 | 0.5 | 0.9 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 210 | 222 | 217 | 197 | 224 | |
| Model 1 | 1 | 1.01 (0.83–1.22) | 0.94 (0.78–1.14) | 0.89 (0.73–1.08) | 0.99 (0.81–1.19) | 0.71 |
| Model 2 | 1 | 1.09 (0.90–1.33) | 1.08 (0.89–1.31) | 1.04 (0.85–1.27) | 1.02 (0.83–1.24) | 0.76 |
| All-cause mortality | ||||||
| Number of deaths | 569 | 541 | 503 | 473 | 434 | |
| Model 1 | 1 | 0.99 (0.88–1.11) | 0.90 (0.80–1.02) | 0.92 (0.81–1.04) | 0.82 (0.72–0.93) | 0.0009 |
| Model 2 | 1 | 1.07 (0.95–1.20) | 1.01 (0.89–1.14) | 1.03 (0.91–1.17) | 0.87 (0.76–0.99) | 0.01 |
| Other vegetables | ||||||
| Median intake, servings/day | 0.3 | 0.5 | 0.7 | 1.0 | 1.5 | |
| Breast cancer-specific mortality | ||||||
| Number of deaths | 210 | 222 | 213 | 221 | 204 | |
| Model 1 | 1 | 1.00 (0.83–1.21) | 0.95 (0.78–1.14) | 0.99 (0.82–1.20) | 0.95 (0.78–1.15) | 0.59 |
| Model 2 | 1 | 1.01 (0.83–1.22) | 0.96 (0.79–1.18) | 0.98 (0.80–1.20) | 0.96 (0.77–1.18) | 0.65 |
| All-cause mortality | ||||||
| Number of deaths | 526 | 545 | 497 | 514 | 439 | |
| Model 1 | 1 | 1.06 (0.94–1.19) | 0.96 (0.85–1.08) | 1.02 (0.90–1.16) | 0.92 (0.81–1.04) | 0.12 |
| Model 2 | 1 | 0.99 (0.88–1.12) | 0.93 (0.82–1.06) | 1.00 (0.88–1.14) | 0.91 (0.79–1.05) | 0.26 |
See Table 2 footnote
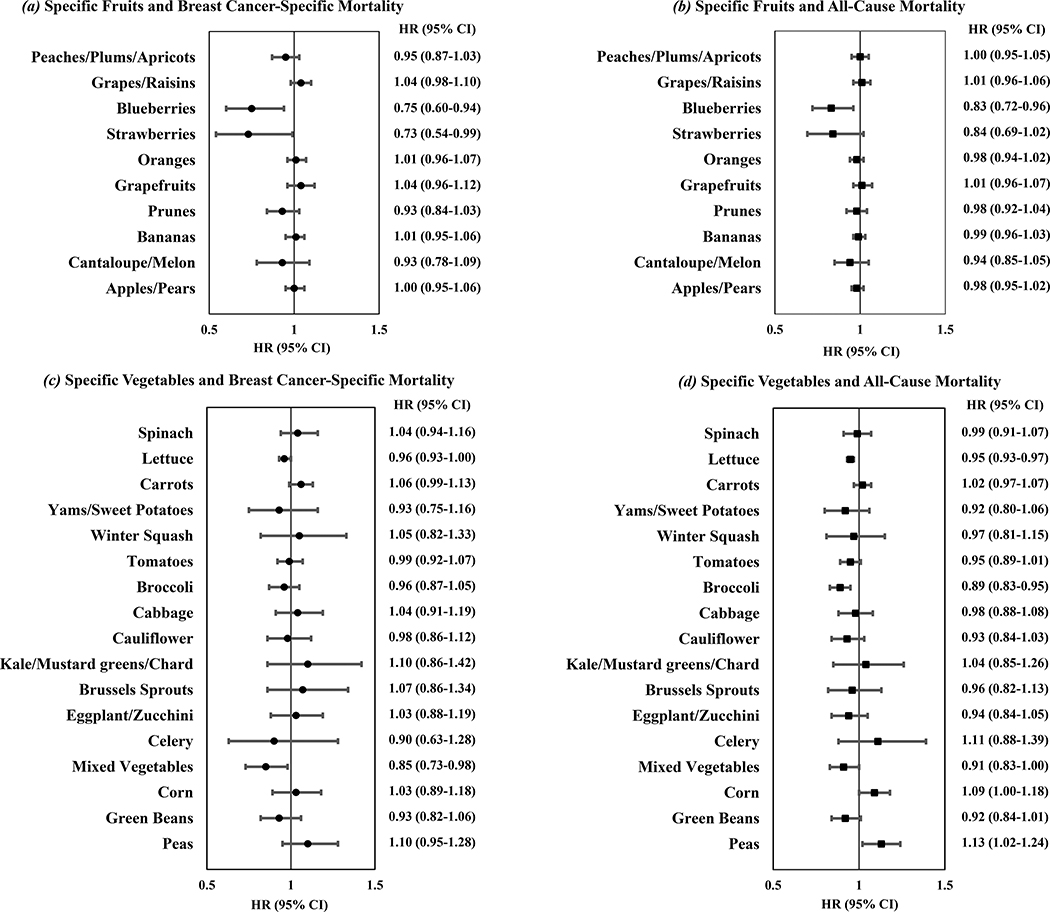
Among individual fruits and vegetables, each 2 servings/week of blueberries after diagnosis was associated with lower breast cancer-specific mortality (HR=0.75, 95%CI=0.60–0.94) and all-cause mortality (HR=0.83, 95%CI=0.72–0.96). Post-diagnostic consumption of strawberries was associated with lower breast cancer-specific mortality (HR=0.73, 95%CI=0.54–0.99), as was mixed vegetable intake (HR=0.85, 95%CI=0.73–0.98). Post-diagnostic broccoli intake was associated with lower risk of all-cause mortality (HR=0.89, 95%CI=0.83–0.95), as was lettuce intake (HR=0.95, 95%CI=0.93–0.97). Post-diagnostic intake of peas was associated with higher all-cause mortality (HR=1.13, 95%CI=1.02–1.24) (Figure 1). The significant association of pea intake was observed even after adjusting for dietary GL.
Figure 1-.
Post-diagnostic specific fruit and vegetable consumption in relation to mortality after breast cancer diagnosis in the NHS and NHSII. Multivariable hazard ratios and 95% confidence intervals are for every 2 servings/week of each fruit or vegetable item.
We also compared the HRs of breast cancer-specific mortality with other causes of mortality. We observed a significant inverse association of total fruit and vegetable, total fruit, and total vegetable consumption with other causes of mortality (for each two servings/day HR=0.88, 95%CI=0.82–0.94, P for heterogeneity=0.05; HR=0.84, 95%CI=0.73–0.97, P for heterogeneity=0.11; and HR=0.84, 95%CI=0.76–0.92, P for heterogeneity=0.12; respectively). Each two servings/day of fruit juice intake were associated with a 30% higher risk of breast cancer-specific mortality (HR=1.30, 95%CI=1.10–1.53), but the association was not significant with other causes of death (HR=1.06, 95%CI=0.92–1.22; P for heterogeneity=0.06).
In exploratory analyses, we examined whether the association between total fruit and vegetable intake and mortality differed by other predictors of cancer prognosis, including age at diagnosis, post-diagnostic smoking status, alcohol consumption, aspirin use, BMI, and physical activity, ER status, and disease stage. Associations were generally similar across all strata, with the exception of age at diagnosis, smoking, and stage of disease, where we observed significant effect modification. Among women younger than 60 years at diagnosis, positive associations were observed between total fruit intake and breast cancer-specific and all-cause mortality. Inverse associations of total fruit and vegetable, total fruit, and total vegetable intakes with all-cause mortality were observed among women ages 60 or more years at diagnosis (Table S4). In addition, the inverse associations of total fruit and vegetable and total vegetable with all-cause mortality were observed among women who were current or past smokers after breast cancer diagnosis. Furthermore, total fruit intake was associated with lower all-cause mortality among women with stage I breast cancer.
We did not observe significant associations of pre-diagnostic intakes of total fruits and vegetables, total fruits, total vegetables, or fruit juice with risk of breast cancer-specific or all-cause mortality (Table S5).
Given dietary intake may change due to recurrences or undiagnosed major illnesses, we performed a 4-year time-lagged analysis. The associations were similar, although somewhat weaker (Table S6).
Finally, associations with the first post-diagnostic measure of fruit and vegetable intake (i.e., without updating) were similar, although somewhat weaker, compared with those using cumulative averages of post-diagnostic food intake (Tables S7).
Discussion
In this analysis, combining cumulatively updated dietary assessments from two large prospective cohorts, we observed lower all-cause mortality with higher intake of total fruits and vegetables, and total vegetables, and subgroups of vegetables including green leafy vegetables, cruciferous vegetables, fruits and vegetables high in vitamin C, and vegetables high in β-carotene. High post-diagnostic fruit juice intake, but not intake of orange juice, was associated with increased risk of death due to breast cancer and all causes. Among individual fruits and vegetables, higher intake of blueberries was significantly associated with lower breast cancer-specific and all-cause mortality. High intakes of strawberries and mixed vegetables were associated with lower risk of breast cancer-specific mortality and high intakes of broccoli and lettuce were associated with lower risk of all-cause mortality.
Fruit and vegetable intake has been hypothesized to improve health in patients with breast cancer, but the overall evidence has been conflicting. A meta-analysis of three prospective studies included earlier data from the NHS (13), Collaborative Women’s Longevity Study (CWLS) (12), and the Women’s Healthy Eating and Living (WHEL) Study (10); with a total of 9,511 cases of breast cancer and 1,218 deaths during follow-up from 5.5–13.1 years (17). Post-diagnostic total fruit or total vegetable consumption was not significantly associated with overall mortality (pooled HR for total fruits=1.04, 95% CI=0.77–1.42; and HR for total vegetables=1.08, 95% CI=0.75–1.55). Our analysis including 8,927 women with breast cancer and 2,521 deaths during up to 30 years of follow-up substantially expands the available evidence on post-diagnostic total fruit and vegetable as well as total vegetable consumption in relation to survival after breast cancer. Considering both pre- and post-diagnostic diet, we observed that the post-diagnostic total fruit and vegetable and total vegetable consumption specifically was associated with better survival. The repeated assessments of diet (up to eight) after diagnosis of breast cancer allowed us to account for changes in diet over time and reduce error in assessing long-term intake. The associations based on the cumulative average of dietary assessments were stronger than when using a single measure of intake, which was a limitation of previous analyses. Given the long survival of the majority of women with breast cancer, the updated diet is a more relevant reflection of exposure over time to examine with respect to survival. Our findings are consistent with a recent report from the Women’s Health Initiative (WHI) Dietary Modification trial indicating total survival benefits for a low-fat dietary pattern without benefits for breast cancer-specific survival (11). As there was an increase in fruit and vegetable consumption in addition to a reported decrease in dietary fat intake between randomized groups within the WHI trial (11), but no change in the fat-sensitive biomarkers (27), the benefit was potentially due to the difference in consumption of fruits, vegetables, and possibly other foods. Our study helps the interpretation of that trial because we were able to adjust for total fat intake and our findings support a benefit for total fruit and vegetable as well as total vegetable consumption independent of fat intake.
Because most deaths among women with breast cancer are not due to breast cancer, both breast cancer-specific and overall survival are important. Substantial evidence supports the benefits of fruit and vegetable consumption on longevity. Our findings with overall survival are consistent with a meta-analysis of prospective studies among healthy persons in which greater total fruit and vegetable consumption was associated with lower all-cause mortality (28).
We observed that high fruit juice consumption after breast cancer diagnosis was associated with worse breast cancer-specific and all-cause mortality. These associations were particularly strong for apple juice, and orange juice was not associated with higher risk. High glycemic index of fruit juices may play an important role in breast cancer survival, however, adjusting for GL or SSB did not account for the associations with fruit juice. These findings merit further investigation in studies which take the nutritional content, which may vary by type and brand of these fruit juices, into account.
Because fruits and vegetables differ greatly in their composition, they would not be expected to have similar relationships with breast cancer survival. This was supported by our analyses. Particularly, post-diagnostic berry consumption may be important in improving survival. The growing in vivo and in vitro evidence indicates that berries and their bioactive components such as phenolic and anthocyanin contents have antiproliferative, anti-inflammatory, and antiangiogenic properties (29) and may inhibit cancer progression, induce apoptosis in breast cancer cells, and reduce tumor growth and metastasis (30–37).
Our results suggest that post-diagnostic cruciferous vegetable consumption may be important in improving overall survival. In the WHEL Study, among patients with breast cancer taking tamoxifen, high post-diagnostic cruciferous vegetable intake was associated with 35% lower breast cancer recurrence (9), but no association was observed in the After Breast Cancer Pooling Project (38), or CWLS (12).
We previously reported that higher intake of vegetables high in β-carotene was associated with a lower incidence of breast cancer, especially tumors with negative ER status (39). Furthermore, in a meta-analysis of seven cohort studies, both higher intake and circulating level of β-carotene were associated with a significant reduced risk of all-cause mortality (highest versus lowest group, pooled relative risk (RR) for β-carotene intake = 0.83, 95%CI: 0.78–0.88; and pooled RR for circulating level of β-carotene= 0.69, 95%CI: 0.59–0.80) among healthy populations (40). This study also supports the role of these foods in reducing all-cause mortality among breast cancer survivors. The association of vegetables high in β-carotene with all-cause mortality was independent of the amount of fiber intake, suggesting other vegetables’ compounds such as carotenoids may play an important role through antioxidant or antiproliferative properties (41).
In contrast, we observed that high intake of peas was associated with higher all-cause mortality. The mechanisms through which peas may increase mortality is unclear. This may be due to their higher content of carbohydrates with a high glycemic index compared to other vegetables, however, the association remained significant after additional adjustment for dietary GL. This finding warrants further investigation in other studies.
The current study has several strengths. Using a prospective design and comprehensive and updated information on pre- and post-diagnostic diet and lifestyle factors allowed us to evaluate the role of fruit and vegetable consumption after diagnosis on breast cancer survival after controlling potential predictors. These analyses also highlighted the strength of using repeated assessments of diet instead of only one diet assessment after diagnosis for evaluating the role of diet on breast cancer survival. Standardized medical record review of reported breast cancer, a wide range of fruit and vegetable consumption, and long duration of follow-up are also strengths of the current study.
The potential limitations also need consideration. As an observational study, this is subject to residual confounding. However, we controlled for many potential confounders, including breast cancer characteristics, treatment, pre-diagnostic fruit and vegetable consumption, and other lifestyle factors associated with survival, including BMI and physical activity. Another limitation of our study is that the findings may not be generalizable to other racial/ethnic groups since the majority of participants were white. Dietary habits may vary according to the seasonal availability of foods and other factors and despite having repeated measures, some misclassification of the exposures is still likely. However, the resulting measurement error is likely to bias the results toward the null, suggesting true associations may be stronger than those observed. Furthermore, we did not inquire about intake of organic fruits or vegetables in the FFQ. Type I error is possible due to multiple comparisons. While chance may play a role, our results are consistent with other literature on overall mortality and specific breast cancer findings, and have biologic plausibility.
In summary, we observed better overall survival after breast cancer diagnosis among women with higher vegetable consumption; specifically, green leafy vegetables as well as cruciferous vegetables, fruits and vegetables high in vitamin C, and vegetables rich in β-carotene. Although eating higher amounts of vegetables may not affect breast cancer-specific mortality, high intake of vegetables and some fruits may improve overall survival. High post-diagnostic fruit juice intake may also be associated with increased risk of death due to breast cancer and all causes, and this finding needs further evaluation.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the NHS and NHSII for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
The authors’ responsibility was as follows: MSF, MDH, WYC, BAR, RMT, WCW, and AHE: designed the research; MSF: analysis and wrote the manuscript; and MSF and AHE: had primary responsibility for the final content of the manuscript; and all authors: provided critical input in the writing of the manuscript and read and approved the final manuscript. The authors assume full responsibility for analyses and interpretation of these data.
FUNDING: The study was supported by the National Institutes of Health Grants (R01 CA050385, UM1 CA176726, UM1 CA186107, P01 CA87969), Breast Cancer Research Foundation, and American Institute for Cancer Research. The study sponsors were not involved in the study design and collection, analysis and interpretation of data, or the writing of the article or the decision to submit it for publication. The authors were independent from study sponsors.
References
- 1.Holmes MD, Wang J, Hankinson SE, Tamimi RM, Chen WE. Protein Intake and Breast Cancer Survival in the Nurses’ Health Study. J Clin Oncol. 2017;35(3):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20(2):167–77. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Trudeau ME, Hood N. Diet and breast cancer: evidence that extremes in diet are associated with poor survival. J Clin Oncol. 2003;21(13):2500–7. [DOI] [PubMed] [Google Scholar]
- 4.Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13(7):1163–72. [PubMed] [Google Scholar]
- 5.Kroenke CH, Kwan ML, Sweeney C, Castillo A, Caan BJ. High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst. 2013;105(9):616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belle FN, Kampman E, McTiernan A, Bernstein L, Baumgartner K, Baumgartner R, et al. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(5):890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izano MA, Fung TT, Chiuve SS, Hu FB, Holmes MD. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr Cancer. 2013;65(6):820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson CA, Rock CL, Thompson PA, Caan BJ, Cussler E, Flatt SW, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women’s Healthy Eating and Living Study. Breast Cancer Res Treat. 2011;125(2):519–27. [DOI] [PubMed] [Google Scholar]
- 10.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Aragaki AK, Anderson GL, Thomson CA, Manson JE, Simon MS, et al. Low-Fat Dietary Pattern and Breast Cancer Mortality in the Women’s Health Initiative Randomized Controlled Trial. J Clin Oncol. 2017;35(25):2919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beasley JM, Newcomb PA, Trentham-Dietz A, Hampton JM, Bersch AJ, Passarelli MN, et al. Postdiagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res Treat. 2011;128(1):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86(5):826–35. [DOI] [PubMed] [Google Scholar]
- 14.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst. 2012;104(24):1905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farvid MS, Eliassen AH, Cho E, Liao X, Chen WY, Willett WC. Dietary Fiber Intake in Young Adults and Breast Cancer Risk. Pediatrics. 2016;137(3):e20151226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, breast cancer survivors. Revised 2018. https://www.aicr.org/wp-content/uploads/2020/01/2014-breast-cancer-survivorship-cup.pdf. [Google Scholar]
- 17.He J, Gu Y, Zhang S. Consumption of vegetables and fruits and breast cancer survival: a systematic review and meta-analysis. Sci Rep. 2017;7(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Lenart E. Reproducibility and validity of food frequency questionnaires. In: Nutritional Epidemiology. Willett WC, ed. New York: Oxford University Press, 2013. p. 96–141. [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134(12):1106–14. [DOI] [PubMed] [Google Scholar]
- 23.Steinmetz KA, Potter JD, Folsom AR. Vegetables, fruit, and lung cancer in the Iowa Women’s Health Study. Cancer Res. 1993;53(3):536–43. [PubMed] [Google Scholar]
- 24.USDA nutrient database for standard reference, release 14: department of agriculture ARS; 2001.
- 25.Holland GWA, Unwin ID, Buss DH, Paul AA, Dat S. The composition of foods: Cambridge UK: The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; 1991. [Google Scholar]
- 26.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–32. [PubMed] [Google Scholar]
- 27.Willett WC. The WHI joins MRFIT: a revealing look beneath the covers. Am J Clin Nutr. 2010;91(4):829–30. [DOI] [PubMed] [Google Scholar]
- 28.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett 2008;269:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaya N, Adams L, Takasaki A, Chen S. Whole blueberry powder inhibits metastasis of triple negative breast cancer in a xenograft mouse model through modulation of inflammatory cytokines. Nutr Cancer. 2014;66(2):242–8. [DOI] [PubMed] [Google Scholar]
- 31.Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF, Wang LS, et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol Nutr Food Res. 2013;57(7):1123–34. [DOI] [PubMed] [Google Scholar]
- 32.Adams LS, Kanaya N, Phung S, Liu Z, Chen S. Whole blueberry powder modulates the growth and metastasis of MDA-MB-231 triple negative breast tumors in nude mice. J Nutr. 2011;141(10):1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wedge DE, Meepagala KM, Magee JB, Smith SH, Huang G, Larcom LL. Anticarcinogenic Activity of Strawberry, Blueberry, and Raspberry Extracts to Breast and Cervical Cancer Cells. J Med Food. 2001;4(1):49–51. [DOI] [PubMed] [Google Scholar]
- 34.Kristo AS, Klimis-Zacas D, Sikalidis AK. Protective role of dietary berries in cancer. Antioxidants 2016;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen V, Tang J, Oroudjev E, Lee CJ, Marasigan C, Wilson L, et al. Cytotoxic effects of bilberry extract on MCF7-GFP-tubulin breast cancer cells. J Med Food. 2010;13(2):278–85. [DOI] [PubMed] [Google Scholar]
- 36.Vuong T, Mallet JF, Ouzounova M, Rahbar S, Hernandez-Vargas H, Herceg Z, et al. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J Transl Med. 2016;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, Raghavan SC. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One. 2012;7(10):e47021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nechuta S, Caan BJ, Chen WY, Kwan ML, Lu W, Cai H, et al. Postdiagnosis cruciferous vegetable consumption and breast cancer outcomes: a report from the After Breast Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farvid MS, Chen WY, Rosner BA, Tamimi RM, Willett WC, Eliassen AH. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int J Cancer. 2019;144(7):1496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao LG, Zhang QL, Zheng JL, Li HL, Zhang W, Tang WG, et al. Dietary, circulating beta-carotene and risk of all-cause mortality: a meta-analysis from prospective studies. Sci Rep. 2016;6:26983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertram JS. Dietary carotenoids, connexins and cancer: what is the connection? Biochem Soc Trans. 2004;32(Pt 6):985–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.