Figure 1.
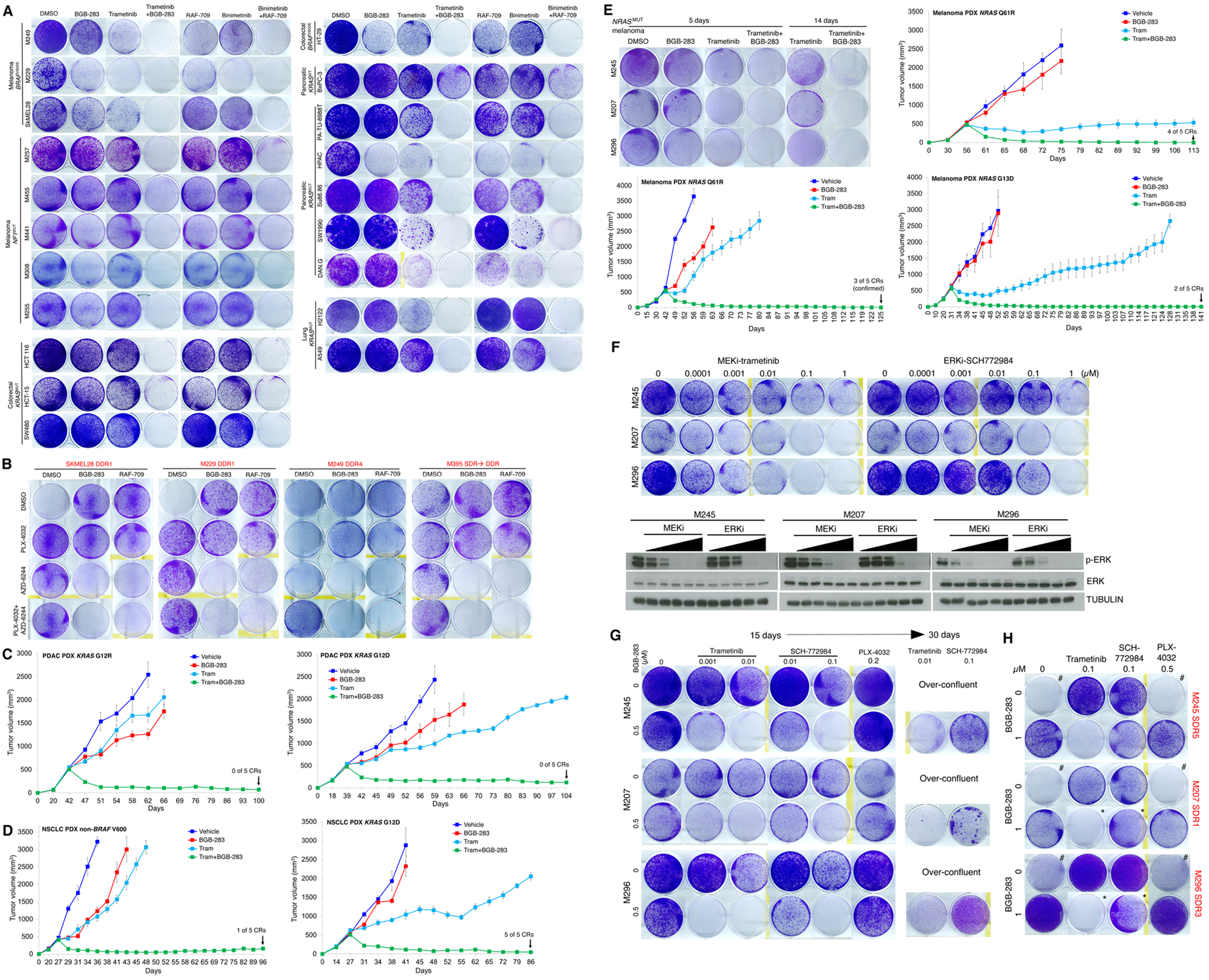
Type II RAFi+MEKi forestalls and overcomes acquired resistance in BRAFMUT, NF1MUT, KRAS MUT and NRAS MUT cancers.
A, Clonogenic growth (14 days) of indicated inhibitor-naïve BRAFV600 or NF1 mutant melanoma, BRAFV600MUT or KRASMUT colorectal carcinoma (CRC), KRASMUT non-small cell lung carcinoma (NSCLC), and KRASMUT (vs. KRASWT) pancreatic ductal adenocarcinoma (PDAC) cell lines after treatment with vehicle (DMSO), BGB-283 (0.5 μM), trametinib (0.02 μM), or trametinib (0.02 μM) plus BGB-283 (0.5 μM) or alternatively with RAF709 (0.5 μM), binimetinib (0.02 μM), or binimetinib (0.02 μM) plus RAF709 (0.5 μM). Data representative of two replicates.
B, Clonogenic growth (15 days, left two sub-lines; 10 days, right two sub-lines) of indicated type I RAFi+MEKi double-drug resistant (DDR; hereafter all names of sub-lines with acquired drug resistance shown in red text) BRAFV600E melanoma sub-lines. DDR sub-lines were maintained on both PLX4032 (1 μM) plus AZD6244 (1 μM), either PLX4032 or AZD6244, or withdrawn from both inhibitors. On top of these four conditions, a type II RAFi (BGB-283 at 1 μM or RAF-709 at 1 μM) was added. Data representative of two replicates.
C, D, Tumor volumes of PDAC (n=2; XWR6, left; XWR7, right, c) and NSCLC (n=2; LBM013-P, left; TM00302, right, d) PDXs in mice that were treated with vehicle, trametinib (3 mg/kg/day PO), BGB-283 (20 mg/kg/day PO), or the combination of trametinib and BGB-283. Vehicle or inhibitor treatments were started at tumor volumes of ~500 mm3. N=5 tumors per group; means ± SEMs.
E, Clonogenic growth (5 or 14 days, upper left panel) of indicated inhibitor-naïve NRASMUT melanoma cell lines after treatment with vehicle (DMSO), BGB-283 (0.5 μM), trametinib (0.02 μM), or trametinib (0.02 μM) plus BGB-283 (0.5 μM). Data representative of two replicates. Tumor volumes of NRASMUT (n=3; NRAS_PDX3, top; NRAS_PDX4, middle; NRAS_PDX1, bottom) PDXs in mice that were treated with vehicle, trametinib (3 mg/kg/day PO), BGB-283 (20 mg/kg/day PO), or the combination of trametinib and BGB-283. Vehicle or inhibitor treatments were started at tumor volumes of ~500 mm3. N=5 tumors per group; means ± SEMs.
F, (Top) Clonogenic growth (10 days) of indicated inhibitor-naïve NRASMUT melanoma cell lines after treatment with vehicle (DMSO) or indicated concentration of MEKi (trametinib) or ERKi (SCH772984). Data representative of two replicates. (Bottom) Western blots of lysates from NRASMUT melanoma cell lines treated (2 hours) with vehicle (DMSO) or the same concentrations of MEKi or ERKi (Top) with indicated antibodies.
G, Clonogenic growth (15 or 30 days) of indicated inhibitor-naïve NRASMUT melanoma cell lines after treatment with vehicle (DMSO) or trametinib, SCH772984, or the type I RAFi PLX-4032 (15 day only) at the indicated concentrations, ± BGB-283. Over-confluent cultures without BGB-283 co-treatment were terminated early and not shown for day 30. Data representative of two replicates.
H, As in G, except NRASMUT melanoma sub-lines (single-drug resistant or SDR, indicated in red) with acquired MEKi (trametinib) resistance were used. Day 15 cultures are shown except those marked with * (which indicates day 30 cultures). Cultures marked with # display the drug addiction phenotype.
