Fig. 7. Neuropathy mutations cause multifaceted disruption of TRPV4–RhoA interactions.
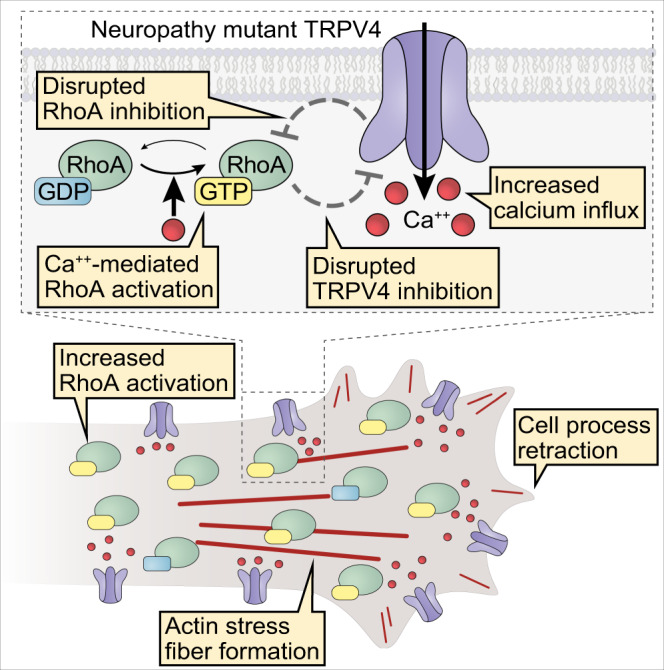
Schematic representation of how TRPV4 neuropathy mutations disrupt normal TRPV4–RhoA functional interactions.TRPV4 binding to RhoA (Fig. 1) is disrupted by TRPV4 neuropathy mutations (Figs. 2 and 3). Impaired binding causes loss of TRPV4-mediated RhoA inhibition (Fig. 4) and disruption of RhoA-dependent TRPV4 ion channel inhibition (Fig. 4). Excessive calcium influx via neuropathy mutant TRPV4 causes further activation of RhoA (Fig. 5). Together, disrupted TRPV4–RhoA interactions lead to increased TRPV4 ion channel activity, increased RhoA activation, RhoA-mediated actomyosin contraction, actin stress fiber formation, and cell process retraction (Figs. 5 and 6).
