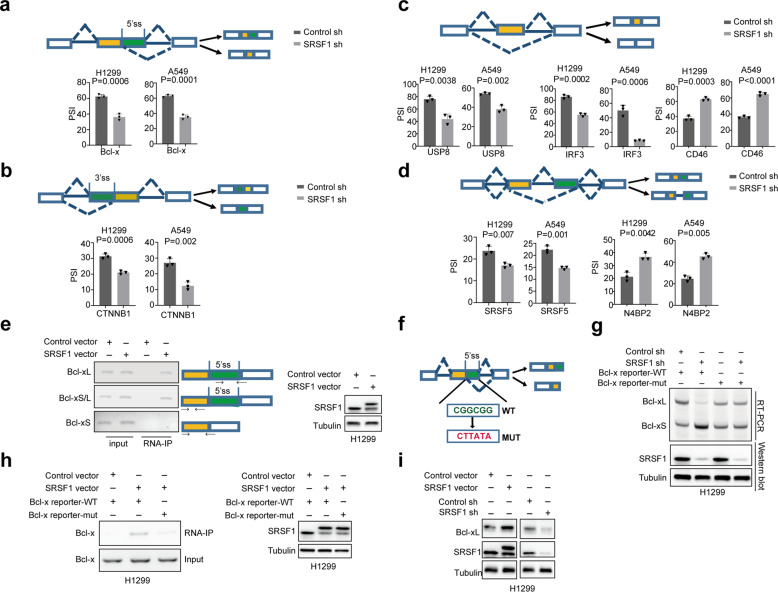
Fig. 2.
SRSF1 controls Bcl-x splicing by directly binding to the pre-mRNAs. a Alternative 5′ splice sites usage of Bcl-x was examined in H1299 and A549 cells with stably reduced SRSF1 or control. b Alternative 3′ splice sites usage of CTNNB1 was determined in H1299 and A549 cells with stable knockdown of SRSF1 or control. c Exon skipping in USP8, IRF3, and CD46 were measured in H1299 and A549 cells with stable decrease of SRSF1 or control. d Intron retention in SRSF5, and N4BP2 were investigated in H1299 and A549 cells with stable reduction of SRSF1 or control. The mean ± SD of PSIs from three experiments were plotted, p values were calculated by paired Student’s t-test in a–d. e Binding of Bcl-x pre-mRNAs with SRSF1 was examined by RNA-immunoprecipitation assay in H1299 cells exogenously expressed FLAG-SRSF1 or control vector. Three pairs primers were used to examine Bcl-xL, Bcl-xS/L, or Bcl-xS, respectively as shown. The protein levels of SRSF1 were measured with western blot. f The schematic of Bcl-x splicing reporters with the predicted SRSF1-binding site in green and the mutated site in red. g Bcl-x splicing reporters containing wild-type or mutated potential SRSF1-binding site were expressed in H1299 cells with stable knockdown of SRSF or control to examine the splicing change of Bcl-x. The protein levels of SRSF1 were also measured. Representative gel and blots were shown. h H1299 cells were co-transfected with Flag-SRSF1 or control vector and the wild-type or mutant Bcl-x reporters, and subsequently immunoprecipitated with anti-Flag antibody. The co-precipitated RNAs were utilized to examine the level of Bcl-x by RT-PCR. A representative gel was demonstrated. The protein levels of SRSF1 were examined with western blot. i The protein levels of Bcl-xL, and SRSF1 were examined in H1299 cells with stable overexpression of SRSF or control vector and stable reduction of SRSF1 or control in a western blot assay