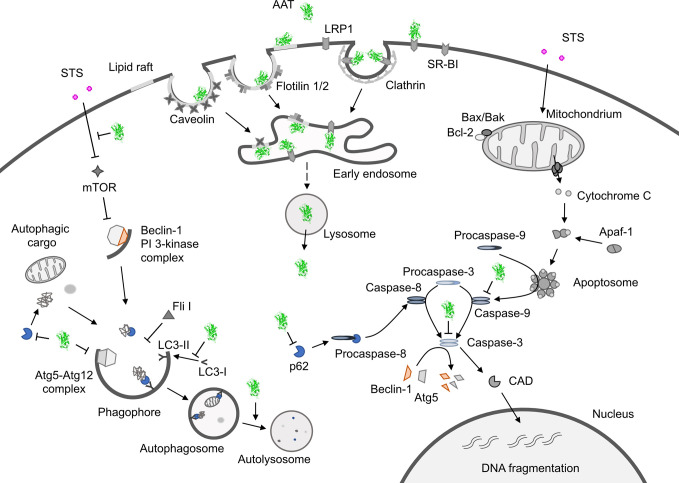
Figure 1.
Hypothetical model showing how alpha1-antitrypsin (AAT) may affect staurosporine (STS)-induced apoptosis and autophagy in NSCLC cells. Intracellular entry of AAT occurs constitutively based on lipid raft independent pathways, namely clathrin-mediated, or lipid rafts-dependent, which include caveolae- and flotilin-dependent endocytosis. The AAT could be sequestered into clathrin-coated pits by binding to LRP1 (low-density lipoprotein receptor related protein 1) and SR-BI (scavenger receptor class b type I). Alternatively, AAT can associate with caveolin or flotilin 1/2-postive lipid rafts. All entry pathways might generate AAT-containing vesicles fusing with early endosomes and lysosomes from which—by unknown mechanisms - AAT escapes into the cytoplasm (green structure). There, AAT could affect cancer cell responses to cytotoxic drugs, like STS (staurosporine). STS activates autophagy by inhibiting mTOR (mammalian target of rapamycin), which allows Beclin-1/PI 3-kinase complex formation, increases LC3-II/LC3-I ratio, p62 degradation in autolysosomes and downregulation of Fli I (Flightless I), a controller of p62-LC3 interaction. Indeed, AAT seems to block different steps required for STS to induce autophagy. Potentially, AAT may also interfere with Atg5-Atg12 complex required for the formation of autophagosomes or recruitment of LC-3 to autophagosomes (green structures). As an inducer of apoptosis, STS acts through the activation of the mitochondrial apoptotic pathway. STS causes the aggregation of Bax/Bak and the release of cytochrome c that binds to Apaf-1 (apoptotic protease-activating factor 1) allowing apoptosome assembly and the recruitment of procaspase-9 to the apoptosome. In this scenario AAT might inhibit activation of procaspases-8, -9, -3 preventing Beclin-1/Atg5 degradation and activation of CAD (caspase-activated DNase). A crosstalk between STS-induced autophagy and apoptosis is in part mediated by p62 and beclin-1. Finding that AAT by itself strongly reduces p62 levels but prevents p62 reduction in STS-treated cells suggests that cancer cells utilize AAT to regulate apoptosis and autophagy dependent on the situation. Under basal conditions, AAT as a reducer of p62 protein levels (also important for the activation of procaspase-8), might activate autophagy as a cytoprotective pathway. In the setting when cancer cells face pro-apoptotic activation, autophagy inhibition may become a strategy to escape apoptosis.