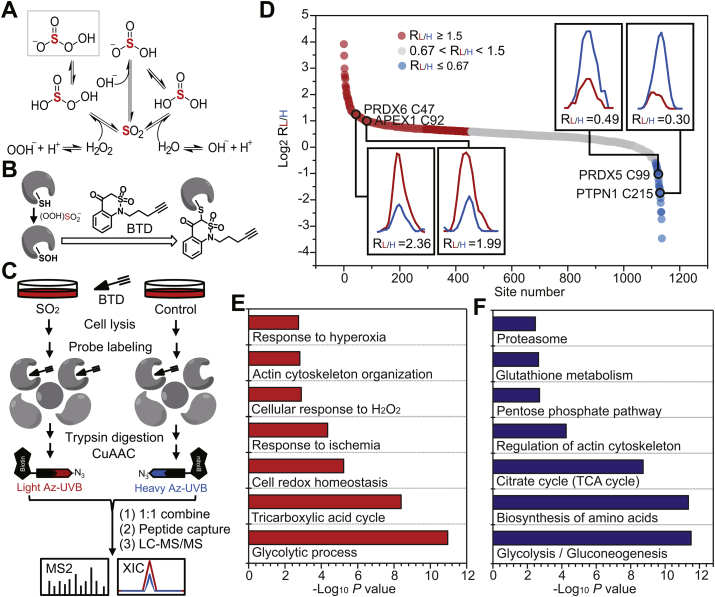
Fig. 2.
SO2 promotes dynamic changes in the sulfenylome of VSMCs. (A) Plausible mechanism of SO2-mediated formation of peroxymonosulfite. Sulfur atom is highlighted in red color. (B) Scheme of chemoselective labeling of protein sulfenic acid by BTD. (C) Schematic workflow for globally and site-specifically profiling protein sulfenylation using the BTD-based SulfenQ method. After 1 h pre-incubation with Ang II, VSMCs were treated with or without 100 μM SO2 donors (SO32‾: HSO3‾ = 2.5:1) for 2 h at 37 °C. Cell lysates were harvested, labeled with 5 mM BTD for 2 h at 37 °C, and then subjected to tryptic digestion. Equal amounts of peptides were reacted with light (for SO2) and heavy (for control) tagged azido-biotin with a photocleavable linker (Az-UV-biotin), respectively. The UV-cleavable biotinylated peptides were captured by streptavidin, and photoleased for LC-MS/MS analysis. (D) Rank order of the determined RSO2/control (RL/H) values of BTD-adducted peptides from VSMCs treated with or without exogenous SO2. GO (E) and KEGG (F) annotations enriched for protein targets of SO2-dependent dynamic sulfenylation. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)