Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide since the end of year 2019 and is currently responsive for coronavirus infectious disease 2019 (COVID-19). The first reports considered COVID-19 as a respiratory tract disease responsible for pneumonia, but numerous studies rapidly emerged to warn the medical community of COVID-19-associated neurological manifestations, including encephalopathy at the acute phase and other postinfectious manifestations. Using standard visual analysis or spectral analysis, recent studies reported electroencephalographic (EEG) findings of COVID-19 patients with various neurological symptoms. Most EEG recordings were normal or revealed non-specific abnormalities, such as focal or generalized slowing, interictal epileptic figures, seizures, or status epilepticus. Interestingly, novel EEG abnormalities over frontal areas were also described at the acute phase. Underlying mechanisms leading to brain injury in COVID-19 are still unknown and matters of debate. These frontal EEG abnormalities could emphasize the hypothesis whereby SARS-CoV-2 enters the central nervous system (CNS) through olfactory structures and then spreads in CNS via frontal lobes. This hypothesis is reinforced by the presence of anosmia in a significant proportion of COVID-19 patients and by neuroimaging studies confirming orbitofrontal abnormalities. COVID-19 represents a new viral disease characterized by not only respiratory symptoms but also a systemic invasion associated with extra-respiratory signs. Neurological symptoms must be the focus of our attention, and functional brain evaluation with EEG is crucial, in combination with anatomical and functional brain imaging, to better understand its pathophysiology. Evolution of symptoms together with EEG patterns at the distance of the acute episode should also be scrutinized.
Keywords: SARS-CoV-2, coronavirus, COVID-19, encephalopathy, neurophysiology, EEG
Introduction
The coronavirus infectious disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, was initially recognized as a respiratory tract disease which could lead to an acute respiratory distress syndrome. However, there is growing evidence of a multi-organ involvement (Gupta et al., 2020). Several authors reported central nervous system (CNS) manifestations, as anosmia referring to olfactory tract involvement. Other critical presentations, including meningoencephalitis, seizures, status epilepticus (SE), encephalopathy, and altered mental status were also described (Ellul et al., 2020). Neurological complications, such as encephalopathy and seizures/SE, and electroencephalographic (EEG) abnormalities, mainly diffuse slowing and epileptiform discharges, have already been described in past viral pandemics such as influenza A H1N1 (Ekstrand et al., 2010; Kedia et al., 2011; Ibrahim and Haddad, 2014). Results of EEG in patients with COVID-19 were increasingly reported. While the volume of COVID-19-related case studies is still growing, we present the spectrum of EEG findings published at the moment, allowing physicians to be cognizant of this new and emerging literature while dealing with COVID-19 patients.
Methods
We considered all studies with EEG findings at the acute phase in COVID-19 patients with neurological manifestations. We performed an electronic research from December 1, 2019, to October 1, 2020, using the database PUBMED by Medline with the following terms (in all fields): (i) (“EEG” OR “electroencephalogram” OR “electroencephalography”) AND (“COVID” OR “coronavirus” OR “SARS-CoV-2”) and (ii) (“brain” OR “nervous system” OR “neurology”) AND (“COVID” OR “coronavirus” OR “SARS-CoV-2”). We also scanned the reference lists of all included articles or relevant reviews for studies to be included in our work. We did not include reviews, non-English articles, unavailable full-text articles, and animal studies. After exclusion of duplicates, we screened the title/abstract or full-text reports and decided whether these met the inclusion criteria.
EEG Observations in COVID-19 Patients
A total of 107 studies were included. Normal EEG findings were reported in adult series (Cecchetti et al., 2020; Helms et al., 2020b; Petrescu et al., 2020) and case reports of patients who displayed various neurological conditions such as focal or generalized seizures (Elgamasy et al., 2020; Fasano et al., 2020; García-Howard et al., 2020; Lyons et al., 2020), non-epileptic seizures (Logmin et al., 2020), myoclonus (Muccioli et al., 2020b; Rábano-Suárez et al., 2020), psychotic symptoms (Lim et al., 2020), encephalopathy (Andriuta et al., 2020; Chaumont et al., 2020; Delorme et al., 2020; Paterson et al., 2020; Perrin et al., 2020), encephalitis (Paterson et al., 2020), brainstem encephalitis (Khoo et al., 2020), and encephalomyelitis (Zoghi et al., 2020). Some studies also reported non-specific abnormalities without more precise EEG features specified by authors (Chougar et al., 2020; Farley and Zuberi, 2020; Freij et al., 2020; Helms et al., 2020a; Pugin et al., 2020).
Diffuse and Focal Slowing
Diffuse slowing of the background activity or focal slowing (sometimes associated with focal sharp waves or epileptiform discharges) was the most frequently published abnormality, especially in adult series (Ayub et al., 2020; Canham et al., 2020; Cecchetti et al., 2020; Chougar et al., 2020; Galanopoulou et al., 2020; Helms et al., 2020a, b; Louis et al., 2020; Pasini et al., 2020; Pellinen et al., 2020; Petrescu et al., 2020; Pilotto et al., 2020a; Scullen et al., 2020; Sethi, 2020; Vespignani et al., 2020) (Figure 1A). Main results of adult series including at least 10 patients with confirmed SARS-CoV-2 infection and EEG recordings are summarized in Table 1. Diffuse or focal slowing was also associated in many case reports with various neurological presentations, mainly of vascular or inflammatory origin. Main vascular complications included ischemic and hemorrhagic strokes (Chaumont et al., 2020; Díaz-Pérez et al., 2020; Morassi et al., 2020; Soldatelli et al., 2020; Zahid et al., 2020), intracranial hemorrhage with cerebral venous thrombosis (Roy-Gash et al., 2020), posterior reversible encephalopathy syndrome (PRES) (Llansó and Urra, 2020; Princiotta Cariddi et al., 2020), intracranial vasculitis (Dixon et al., 2020), subarachnoid hemorrhage (Harrogate et al., 2020), acute hemorrhagic leukoencephalitis or leukoencephalomyelitis (Handa et al., 2020; Kihira et al., 2020; Svedung Wettervik et al., 2020), and acute necrotizing encephalopathy (Delamarre et al., 2020; Virhammar et al., 2020). Main inflammatory syndromes included acute disseminated encephalomyelitis (ADEM) (Parsons et al., 2020; Umapathi et al., 2020), acute leukoencephalopathy (Abenza-Abildúa et al., 2020; Anand et al., 2020; Brun et al., 2020; Huang H. et al., 2020; Kihira et al., 2020; Klironomos et al., 2020), acute leukoencephalitis (Perrin et al., 2020), meningoencephalitis without any acute lesions on brain imaging (Duong et al., 2020; El-Zein et al., 2020; Pilotto et al., 2020b), Bickerstaff encephalitis (Llorente Ayuso et al., 2020), and concomitant autoimmune encephalitis (Grimaldi et al., 2020; Panariello et al., 2020). In critically ill patients, other conditions were described including post-hypoxic injury (Fischer et al., 2020; Radmanesh et al., 2020; Radnis et al., 2020; Vellieux et al., 2020), unresponsiveness after sedation discontinuation (Espinosa et al., 2020; Vellieux et al., 2020), encephalopathy or altered mental status without any acute lesions on brain imaging (Chaumont et al., 2020; Delorme et al., 2020; Filatov et al., 2020; Gaughan et al., 2020; Jang et al., 2020; Manganelli et al., 2020; Muccioli et al., 2020a; Méndez-Guerrero et al., 2020; Romero-Sánchez et al., 2020; Shekhar et al., 2020), encephalopathy with seizures (Ashraf and Sajed, 2020; Benameur et al., 2020; Farhadian et al., 2020; Haddad et al., 2020), defined toxic/metabolic encephalopathy (Flamand et al., 2020; Radmard et al., 2020; Rasmussen et al., 2020), neuroleptic malignant syndrome (Kajani et al., 2020), after seizures or SE (Anand et al., 2020; Edén et al., 2020; Emami et al., 2020), and critical illness-associated cerebral microbleeds (De Stefano et al., 2020). EEG slowing was also observed in pediatric reports (Abdel-Mannan et al., 2020; Abel et al., 2020; Dugue et al., 2020; Panda et al., 2020).
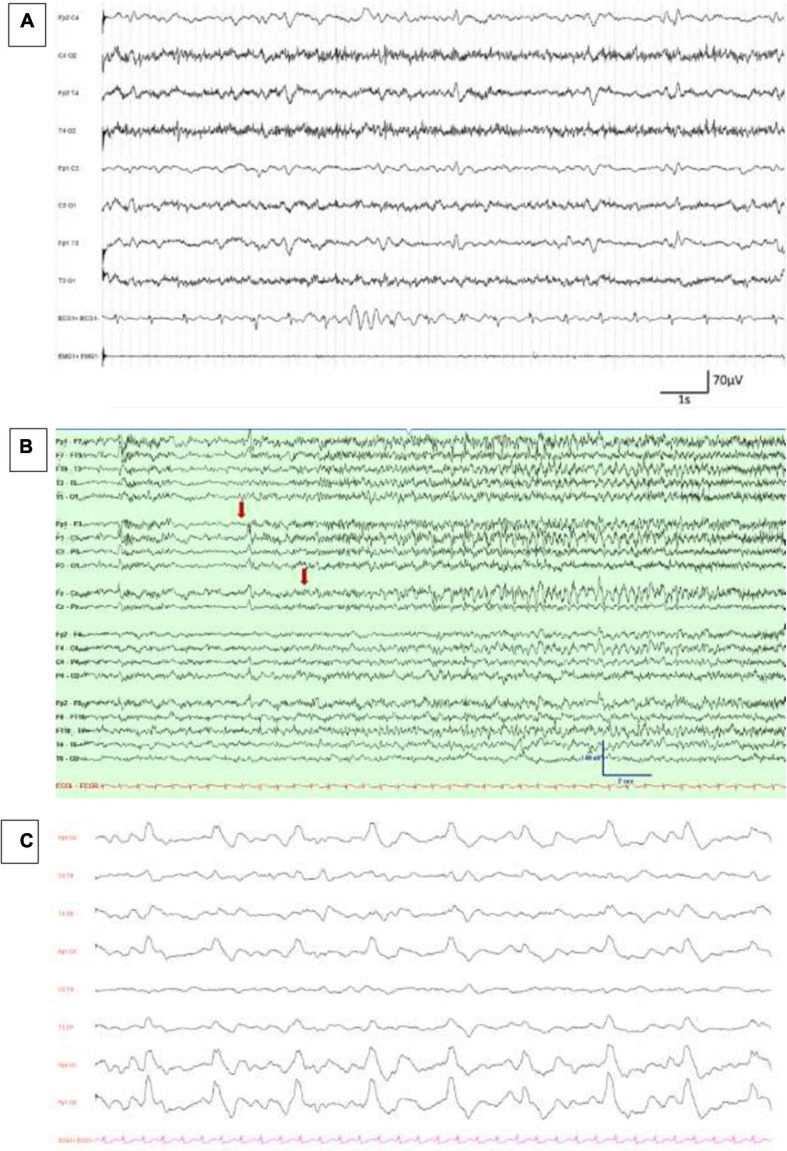
FIGURE 1.
EEG findings in COVID-19 patients. (A) Diffuse theta–delta slowing and continuous generalized periodic discharges, reproduced with authors’ agreement from Petrescu et al. (2020). (B) Emergence of low-amplitude ictal fast rhythmic activity over left frontocentral and midline regions (marked with an arrow), reproduced with authors’ agreement from Somani et al. (2020). (C) Continuous, periodic, monomorphic diphasic, delta slow waves over both frontal areas, published in Vellieux et al. (2020).
TABLE 1.
Main results of case series including at least 10 patients admitted for COVID-19 with EEG recordings.
| Series | Patients’ features | Brain imaging results | CSF results | EEG settings | Ongoing psychoactive drugs | Main EEG results |
| Ayub et al., 2020 | >Included, n = 37 | > CT-scan, n = 35 and MRI, n = 9 | >CSF examination, n = 4 | >Total EEG recordings, n = 37 | >At time of EEG or the day prior | >Background activity |
| USA | M/F, n = 27/10 | IS, n = 3 | Abnormal WBC count, n = 2 | Propofol, n = 19 | Absent PDR, n = 34 | |
| Monocentric | Median age: 66 years | ICH, n = 3 | Abnormal protein level, n = 1 | >Types of EEG | Dexmedetomidine, n = 13 | Asymmetry, n = 4 |
| Anosmia, n = 4 | Long-term monitoring EEG, n = 23 | Empiric AD, n = 11 | Generalized delta and theta slowing, n = 34 | |||
| Intubated, n = 28 | Midazolam or lorazepam, n=8 | Burst suppression, n=5 | ||||
| >Period of recordings: NA | Ketamine, n=2 | Unreactive, n=1 | ||||
| Prior neurological history | ||||||
| Stroke, n = 6 | >EEG indication | >Rhythmic and periodic patterns | ||||
| Cerebral aneurysm, n = 1 | Altered mental status, n=24 | GPDs without triphasic waves, n=4 | ||||
| Epilepsy, n = 1 | Possible seizures, n=11 | GPDs with triphasic waves, n=8 | ||||
| ICH, n = 1 | Cardiac arrest, n=2 | SIRPIDs, n=3 | ||||
| DLB, n = 1 | GRDA, n=5 | |||||
| LRDA, n=1 | ||||||
| >Epileptiform findings and seizures | ||||||
| Burst suppression with epileptiform activity, n=4 | ||||||
| Focal sporadic discharges, n=1 | ||||||
| Multifocal sporadic discharges, n=6 | ||||||
| Generalized sporadic discharges without triphasic waves, n=8 | ||||||
| Generalized NCSE, n=1 | ||||||
| Canham et al., 2020 | >Included, n=10 | >CT-scan, n=10 | >CSF examination, n=6 | >Total EEG recordings, n=11 | >At time of EEG | >Background activity |
| United Kingdom | M/F, n=8/2 | Normal, n=3 | Abnormal WBC count, n=3 | Levetiracetam, n=6 | Generalized symmetrical slowing, n=11 | |
| Multicentric | Median age: 65 years | Small vessel disease, n=4 | Abnormal protein level, n=4 | >Types of EEG | Propofol, n=2 | Anterior emphasis of slow activity, n=3 |
| Anosmia/agueusia: NA | SAH, n=2 | Negative HSV 1&2, VZV | 9 electrodes 20-30 min EEG, n=11 | Alfentanil, n=2 | Asymmetry, n=1 | |
| Intubated: NA | Atrophy, n=2 | and enterovirus PCR, n=6 | Phenytoin, n=2 | |||
| Negative SARS-CoV-2 PCR, n=2 | >Period of recordings: NA | Valproate, n=2 | >Rhythmic and periodic patterns | |||
| Prior neurological history | >MRI, n=4 | Lamotrigine, n=1 | FIRDA, n=1 | |||
| SAH, n=1 | Normal, n=1 | >EEG indication | Gabapentin, n=1 | |||
| Stroke, n=1 | Small vessel disease, n=2 | Altered mental status, n=6 | Carabamazepine, n=1 | >Epileptiform findings and seizures, n=0 | ||
| Learning difficulties, n=1 | IS, n=1 | Seizure, n=6 | Lacosamide, n=1 | |||
| Essential tremor, n=1 | Atrophy, n=1 | Delirium, n=2 | Primidone, n=1 | |||
| Epilepsy, n=1 | Amitriptyline, n=1 | |||||
| Lorazepam, n=1 | ||||||
| Citalopram, n=1 | ||||||
| Olanzapine, n=1 | ||||||
| Clozapine, n=1 | ||||||
| Paliperidone, n=1 | ||||||
| Midazolam, n=1 | ||||||
| Remifentanyl, n=1 | ||||||
| Morphine, n=1 | ||||||
| Cecchetti et al., 2020 | >Included, n=18 | >CT-scan and/or MRI | >CSF examination, n = 1 | >Total EEG recordings, n=18 | NA | >Background activity |
| Italy | M/F, n=11/7 | PRES, n=1 | Normal WBC count, n=1 | Normal or with mild alteration, n=5 | ||
| Monocentric | Mean age: 67 years | Remote ICH, n=1 | Normal protein level, n=1 | >Types of EEG | With moderate alteration, n=9 | |
| Anosmia/agueusia, n=0 | Remote IS, n=1 | Negative bacteriologic and | Basal EEG, n=18 | With severe alteration, n=4 | ||
| Intubated: NA | Glioblastoma, n=1 | virologic assays (including | Generalized slowing, n=16 | |||
| Metastasis, n=1 | SARS-CoV-2 RT-PCR), n=1 | >Period of recordings: NA | Anterior (bifrontal) prevalence of slow waves, n=10 | |||
| Prior neurological history | Traumatic SDH, n=1 | Focal slowing, n=7 | ||||
| NA | Anterior pontine demyelinating lesion, n=1 | >EEG indication | ||||
| Transient loss of consciousness, n=5 | >Rhythmic and periodic patterns, n=0 | |||||
| Seizures/spasms, n=5 | ||||||
| Coma, n=5 | >Epileptiform findings and seizures | |||||
| Delirium, n=3 | Epileptiform discharges, n=2 | |||||
| Seizures, n=0 | ||||||
| Chougar et al., 2020 | >Included, n=73 | >MRI, n=73 | >CSF examination, n=39 | >Total EEG recordings, n=40 | NA | >Background activity / Epileptiform findings and seizures |
| France | M/F, n=48/25 | No significant abnormalities, n=30 | Abnormal WBC count, n=8 | Pathological findings related to seizure or encephalopathy, n=9 | ||
| Monocentric | Mean age: 56 years | Acute IS, n=17 | Abnormal protein level, n=10 | >Types of EEG: NA | Non-specific findings, n=24 | |
| Anosmia/agueusia, n=4 | Multiple microhemorrhages, n=8 | Oligoclonal bands, n=2 | ||||
| Intubated: NA | Multifocal enhancing WM lesions, n=4 | Negative bacteriologic and | >Period of recordings: NA | >Rhythmic and periodic patterns: NA | ||
| Basal ganglia lesions, n=4 | virologic assays (including | |||||
| Prior neurological history | Hypoxic-ischemic lesions, n=3 | HSV 1&2, VZV, CMV, EBV | >EEG indication: NA | |||
| Stroke, n=NA | Cytotoxic lesions of the CC, n=3 | and SARS-CoV-2 RT-PCR), n=39 | ||||
| Central pontine myelinolysis, n=3 | ||||||
| PRES, n=2 | ||||||
| Meningeal enhancement, n=2 | ||||||
| Neuritis, n=2 | ||||||
| Deep venous thrombosis, n=1 | ||||||
| Corticospinal tracts FLAIR hyperintensity, n=1 | ||||||
| >Perfusion MRI, n=46 | ||||||
| Seizure-related perfusion abnormalities, n=9 | ||||||
| Recent or old vascular lesions-related perfusion abnormalities, n=4 | ||||||
| Perfusion abnormalities unrelated to seizures or ischemia, n=10 | ||||||
| Galanopoulou et al., 2020 | >Included, n=22 | >Modality: NA (at least 1 brain MRI) | NA | >Total EEG recordings, n=31 | >During hospital stay (at time of EEG: NA) | >Background activity |
| USA | M/F, n=14/8 Mean age: 63 years Anosmia/agueusia: NA Intubated, n=14 | Subcortical and mild periventricular WM signal | Sedatives, n=14 | Bilateral slowing, n=22 Focal slowing, n=5 Asymmetry, n=3 Absent PDR, n=18 Slow PDR, n=4 Discontinuous or burst suppression, n=1 | ||
| Multicentric | hyperintensity, n = 1 | >Types of EEG | AD, n=12 | |||
| SAH due to aneurysm, n=1 | 10 electrodes/8-channel EEG, n=20 | |||||
| SDH, n=1 | Routine EEG, n=4 | |||||
| cEEG, n=7 | ||||||
| Prior neurological history | ||||||
| Epilepsy, n=4 | >Period of recordings: NA | |||||
| Neurological disorders except epilepsy, n=7 | >Rhythmic and periodic patterns | |||||
| >EEG indication | Generalized or frontal RDA, n=3 | |||||
| Altered mental status, n=20 | Temporal LRDA, n=1 | |||||
| Motor seizure-like event or seizure | Bifrontal sharply contoured periodic waves, n=1 | |||||
| at presentation or confusion | ||||||
| resembling prior seizures, n=12 | >Epileptiform findings and seizures | |||||
| Gaze deviation, n=2 | Bilateral frontal sharp waves, n=6 | |||||
| Confusion at presentation and no | Unilateral frontal sharp waves, n=2 | |||||
| prior seizures, n=1 | Temporal or hemispheric sharp waves, n=2 | |||||
| Seizures, n=0 | ||||||
| Helms et al., 2020a | >Included, n=58 | >MRI, n=13 | >CSF examination, n=7 | >Total EEG recordings, n=8 | >During hospital stay (at time of EEG: NA) | >Background activity |
| France | M/F: NA | Leptomeningeal enhancement, n=8 | Normal WBC count, n=7 | Sufentanil, n=58 | Nonspecific changes, n=8 | |
| Bicentric | Median age: 63 years | Acute IS, n=2 | Elevated protein level, n=1 | >Types of EEG: NA | Midazolam, n=50 | Diffuse bifrontal slowing, n=1 |
| Anosmia/agueusia: NA Intubated, n=58 | Subacute IS, n=1 | Oligoclonal bands with mirror pattern, n=2 | >Period of recordings: NA | Propofol, n=27 | >Rhythmic and periodic patterns: NA | |
| >Perfusion MRI, n=11 | Negative SARS-CoV-2 RT-PCR, n=7 | >EEG indication: NA | ||||
| Prior neurological history | Bilateral frontotemporal hypoperfusion, n=11 | >Epileptiform findings and seizures: NA | ||||
| TIA, partial epilepsy, MCI, n=7 | ||||||
| Helms et al., 2020b | >Included, n=140 | >MRI, n=28 | >CSF examination, n=25 | >Total EEG recordings, n=42 | >During hospital stay (at time of EEG: NA) | >Background activity |
| France | M/F, n=100/40 | Subarachnoid spaces FLAIR and T1 contrast | Elevated WBC count, n=3 | Midazolam, n=121 | Normal, n=5 | |
| Bicentric | Median age: 62 years Anosmia/agueusia: NA Intubated, n=140 Prior neurological history Stroke/TIA, n=9 Migraine, n=5 Mild cognitive alteration, n=4 Partial epilepsy, n=2 Trauma brain injury, n=2 Aneurysm, n=1 | enhancement, n=17 WM microhemorrhages, n=7 WM FLAIR hyperintensities, n=4, with small foci of contrast enhancement, n=2 and diffusion hyperintensities, n=2 Acute IS, n=2 Intraparenchymal hematoma, n=1 Preexisting IS, n=1 >Perfusion MRI, n=26 Perfusion abnormalities, n=17 | Elevated protein level, n=8 Elevated IgG levels, n=9 Oligoclonal bands with mirror pattern, n=13 Positive SARS-CoV-2 RT-PCR (negative result in blood), n=1 Negative bacterial cultures and viral research (HSV 1&2, enterovirus), n = 25 | >Types of EEG: NA >Period of recordings: NA > EEG indication Unexplained and persistent alteredconsciousness after prolongedsedation discontinuation (> 3 days) Multimodality neurologicalscreening in combination with brainMRI and/or CSF examination | Sufentanil, n=138 Propofol, n=83 | Unspecific abnormalities, with low voltage, rapid rhythm, and lack of asymmetry, n=26 Diffuse, especially bifrontal, slow activity n=11 >Rhythmic and periodic patterns: NA > Epileptiform findings and seizures: NA |
| Louis et al., 2020 | >Included, n=22 | >CT-scan, n=18 | NA | >Total EEG recordings, n=22 >Types of EEG cEEG, n=19 Routine EEG, n=3 | >At time of EEG | >Background activity |
| USA Monocentric | M/F, n=14/8 | Possible IS, n=2 | Sedative drugs (including fentanyl, propofol and/or midazolam), n=14 | Continuous generalized polymorphic delta slowing, n=19 | ||
| Mean age: 67 years | Acute IS, n=1 | Slow PDR, n=9 | ||||
| Anosmia/agueusia: NA | ICH, n=1 | Absent PDR, n=11 | ||||
| Intubated, n=18 | Normal PDR, n=2 | |||||
| >MRI, n=1 | ||||||
| Prior neurological history | Acute IS, n=1 | >Period of recordings: NA | >Rhythmic and periodic patterns | |||
| Epilepsy, n=2 | GPDs, n=7 | |||||
| Stroke, n=1 | >EEG indication | GPDs with triphasic morphology, n=5 | ||||
| Headache, n=1 | Altered mental status, n=17 | GPDs with sharply contoured morphology, n=2 | ||||
| Traumatic brain injury, n=1 | Seizure-like event, n=5 | Intermittent GRDA, n=11 | ||||
| Spinal stenosis, n=1 | Hemispheric LRDA, n=1 | |||||
| >Epileptiform findings and seizures | ||||||
| Epileptic abnormalities, n=5 | ||||||
| Seizures, n=2 | ||||||
| Pasini et al., 2020 | >Included, n=15 | >CT-scan, n=8 | >CSF examination, n=5 | >Total EEG recordings, n=15 | NA | Subset of non post-anoxic patients, n=13 |
| Italy | M/F, n=6/9 | Normal, n=8 | Elevated protein level, n=1 | >Background activity | ||
| Monocentric | Mean age: 65 years | Negative SARS-CoV-2 detection, n=5 | >Types of EEG | Generalized slowing with theta prevalence, n=5 | ||
| Anosmia/agueusia: NA | >MRI, n=6 | 18 electrodes EEG, n=15 | Generalized slowing with intrusions of theta/delta activity, n=4 | |||
| Intubated: NA | Mild WM T2 hyperintensity, n=2 | >Period of recordings: NA | Focal slowing predominantly over the frontal or central regions n=3 | |||
| Unreactive, n=10 | ||||||
| Prior neurological history | ||||||
| Cognitive decline, n=2 | >EEG indication | >Rhythmic and periodic patterns | ||||
| Limbic encephalitis, n=1 | Confusion, n=11 | FIRDA, n=1 | ||||
| Frontal metastasis, n=1 | Impairment of consciousness, n=4 with post-anoxic coma, n=2 | >Epileptiform findings and seizures | ||||
| Aphasia, n = 1 | Epileptiform abnormalities, n=0 | |||||
| Subset of post-anoxic comas, n=2 | ||||||
| Severely suppressed activity, n=1 | ||||||
| Discontinued activity compatible with post-anoxic SE, n=1 | ||||||
| Unreactive, n=2 | ||||||
| Pellinen et al., 2020 USA Multicentric | >Included, n=111 M/F, n=79/32 Median age: 64 years Anosmia/agueusia: NA Intubated, n=79 Prior neurological history Stroke, n=23 Epilepsy, n=13 ICH, n=4 Dementia, n=4 Developmental delay/intellectual disability, n=3 Brain tumor, n=3 Traumatic brain injury, n=2 Parkinson disease, n=2 Vascular malformation, n=1 Tuberous sclerosis complex, n=1 Herpes encephalitis, n=1 | >Brain imaging, n=90 (with CT-scan only, n=75) Acute IS, n=18 Acute ICH, n=15 Cerebral edema, n=6 Diffuse leukoencephalopathy with microhemorrhages, n = 4 Mixed acute ischemic and hemorrhagic lesions, n=3 | NA | >Total EEG recordings, n=118 >Types of EEG 21-channel cEEG for a target of at least 24 hours, n=111 Rapid EEG system with 8-bipolar channel montage 0.5-12 hours, n=7 >Period of recordings: NA >EEG indication Persistent encephalopathy, n=72 Paroxysmal activity of unclear cause, n=25 Seizure exacerbation, n=10 Cardiac arrest n=11 | >During EEG Sedative drugs (including propofol, midazolam, pentobarbital, dexmedetomidine and/or fentanyl) n=67 >Prior to EEG AD, n=57 | >Background activity Normal, n=5 Mild generalized slowing, n=17 Moderate generalized slowing, n=60 Severe generalized slowing/discontinuous/ECI, n=29 Focal slowing, n=27 >Rhythmic and periodic patterns GRDA, n=4 LRDA, n=7 LRDA and GRDA, n=2 GPDs, n=11 LPDs, n=3 >Epileptiform findings and seizures Focal epileptiform discharges, n=12 Multifocal epileptiform discharges, n=6 Generalized epileptiform discharges, n=5 Seizures, n=8 NCSE, n=2 |
| Petrescu et al., 2020 | >Included, n=36 | >CT-scan, n=14 | >CSF examination, n=4 | >Total EEG recordings, n=40 | >At time of EEG | >Background activity |
| France | M/F, n=26/10 | Normal, n=4 | Normal, n=4 | Levetiracetam, n=6 | Normal, n=4 | |
| Monocentric | Mean age: 70 years | Atrophy, n=9 | >Types of EEG | Sedations, n=5 | Mildly altered, n=19 | |
| Anosmia/agueusia: NA | IS, n=2 | Routine 20 min EEG, n=40 | Risperidone, n=4 | Moderately altered, n=4 | ||
| Intubated, n=11 | Calcification, n=2 | Clobazam, n=2 | Severely altered, n=8 | |||
| SDH, n=1 | >Period of recordings: NA | Dexmedetomidine, n=2 | Critically altered, n=5 | |||
| Prior neurological history | Leukoaraiosis, n=1 | Citalopram or escitalopram, n=2 | Focal bioccipital slowing, n=1 | |||
| Dementia, n=10 | Meningioma, n=1 | >EEG indication | Midazolam, n=2 | Sporadic triphasic waves, n=1 | ||
| Stroke, n=3 | Postoperative lesion, n=1 | Fluctuating alertness, n=13 | Oxazepam, n=2 | |||
| SDH, n=2 | Confusion, n=9 | Morphine, n=2 | >Rhythmic and periodic patterns | |||
| Memory impairment, n=1 | >MRI, n=11 | Delayed awakening after stopping | Oxazepine, n=1 | RDA, n=7 with frontal predominant, n=1 | ||
| Hydrocephalus, n=1 | Atrophy, n=4 | sedation or inadequate emerge of | Haloperidol, n=1 | GPDs, n=6 | ||
| Epilepsy, n=1 | IS, n=2 | sedation, n=8 | Doxylamine succinate, n=1 | Multifocal PDs, n=2 | ||
| Parkinson disease, n=1 | SDH, n=2 | Focal neurologic symptoms, n=6 | Lacosamide, n=1 | |||
| Gliosis of CC, n=1 | Seizures, n=3 | Diazepam, n=1 | >Epileptiform findings and seizures | |||
| Leukoaraiosis, n=1 | Abnormal movements, n=3 | Valproate, n=1 | Epileptiform discharges, n=0 | |||
| Leptomeningeal enhancement, n=1 | Cardiac arrest, n=1 | Bromazepam, n=1 | Seizures, n=0 | |||
| Probable septic lesions (multiple ischemic and hemorrhagic lesions) related to endocarditis, n=1 | Encephalopathy, n=1 | Gabapentin, n=1 | ||||
| Control follow-up, n=1 | Paroxetine, n=1 | |||||
| Multiple FLAIR hyperintense lesions, n=1 | Alprazolam, n=1 | |||||
| Hydroxyzine, n=1 | ||||||
| Mianserine, n=1 | ||||||
| Pilotto et al., 2020a Italy Multicentric | >Included patients, n=25 M/F, n=15/10 Mean age: 66 years Anosmia/agueusia: NA Intubated, n=4 Prior neurological history Stroke, n=2 Mental retardation, n=1 Possible encephalitis and Behçet disease, n=1 | >MRI, n = 25 Normal, n=13 Multiple subcortical T2-hyperintensities, n=4 Focal cortical T2 and DWI hyperintensities, n=3 Acute necrotizing encephalopathy, n=2 Limbic encephalitis, n=2 ADEM, n=1 Leptomeningeal enhancement, n=1 | >CSF examination, n=25 Normal, n=8 Elevated WBC count, n=9 Elevated protein level, n=15 Negative bacteriological and virological screening, n=25 Negative SARS-CoV-2 RT-PCR, n=14 | >Total EEG recordings, n=25 >Types of EEG: NA >Period of recordings: NA >EEG indication Delirium/altered mental status, n=17 Aphasia/dysarthria, n=6 Seizures, n=6 | NA | >Background activity Generalized slowing especially localized to frontal derivations, n=16 >Rhythmic and periodic patterns: NA >Epileptiform findings and seizures Focal epileptic alterations, n=6 |
| Scullen et al., 2020 | >Included patients, n=27 | >CT-scan, n=27 | NA | >Total EEG recordings, n=13 | NA | >Background activity |
| USA Monocentric | M/F, n=14/13 | Focal hypodensities in deep structures, n=14 | Generalized encephalopathy (i.e. irregular slowing with delta and theta frequency oscillations), n=11 | |||
| Mean age: 60 years | Diffuse hypoattenuation, n=6 | >Types of EEG | ||||
| Anosmia/agueusia, n=1 | Subacute IS, n=4 | cEEG, n=13 | >Rhythmic and periodic patterns: NA | |||
| Intubated: NA | Subcortical parenchymal hematoma, n=3 | |||||
| >Period of recordings: NA | >Epileptiform findings and seizures | |||||
| Prior neurological history | >MRI, n=8 | >EEG indication | NCSE, n=1 | |||
| Stroke, n=3 | Viral encephalitis with diffuse involvement of the | Pronounced encephalopathy not explained by previous CT alone, n=9 | ||||
| Pseudotumor cerebri, n=1 | deep WM, CC and basal ganglia, n=NA | Pronounced encephalopathy not explained by previous combined CT and MRI, n=4 | ||||
| Sethi, 2020 | NA | NA | NA | >Total EEG recordings, n=20 | NA | >Background activity |
| USA | Diffuse theta and delta slowing | |||||
| Monocentric | >Types of EEG: NA | |||||
| >Rhythmic and periodic patterns: NA | ||||||
| >Period of recordings: NA | ||||||
| >Epileptiform findings and seizures: NA | ||||||
| >EEG indication | ||||||
| Altered mental status | ||||||
| Vespignani et al., 2020 | >Included patients, n=26 | >CT-scan, n=1 | >CSF examination, n=2 | >Total EEG recordings, n=26 | Subset of the 5 patients with PDs | >Background activity |
| France | Occipital cyst, n=1 | Normal, n=2 | >At time of EEG | Diffuse slowing without PDs, n=19 | ||
| Multicentric | Subset of patients with PDs, n=5 | >Types of EEG | Propofol, n=2 | Isoelectric, n=2 | ||
| M/F, n=4/1 | >MRI, n=1 | 9 electrodes 30 min EEG, n=26 | Fentanyl, n=2 | |||
| Mean age: 67 years | Diffuse WM hyperintensities, n=1 | Midazolam, n=1 | >Rhythmic and periodic patterns | |||
| Anosmia/agueusia: NA | >Period of recordings: NA | GPDs with frontal involvement, n=4 | ||||
| Intubated, n=4 | LPDs with frontal involvement, n=1 | |||||
| Prior neurological history: NA | >EEG indication | |||||
| Mental status changes | >Epileptiform findings and seizures, n=0 | |||||
| Poor responsiveness | ||||||
| Determine the presence of SE in non-arousable patients | ||||||
| Subset of the 5 patients with PDs | ||||||
| Poor or absent responsiveness, n=4 | ||||||
| Cardiac arrest, n=1 | ||||||
| Confusion and lethargy, n=1 | ||||||
AD: antiepileptic drug, ADEM: acute disseminated encephalomyelitis, CC: corpus callosum, cEEG: continuous EEG, CMV: cytomegalovirus, CSF: cerebrospinal fluid, CT: computed tomography, DLB: dementia with Lewy bodies, DWI: diffusion weighted imaging, EBV: Epstein-Barr virus, ECI: electrocerebral inactivity, EEG: electroencephalogram, FIRDA: frontal intermittent rhythmic delta activity, FLAIR: fluid-attenuated inversion recovery, GPDs: generalized periodic discharges, GRDA: generalized rhythmic delta activity, HSV: herpes simplex virus, ICH: intracranial hemorrhage, IS: ischemic stroke, LRDA: lateralized rhythmic delta activity, MCI: mild cognitive impairment, M/F: male/female, n: number, MRI: magnetic resonance imaging, NA: not available, NCSE: non convulsive SE, PCR: polymerase chain reaction, PDR: posterior dominant rhythm, PDs: periodic discharges, PRES: posterior reversible encephalopathy syndrome, RDA: rhythmic delta activity, RT-PCR: reverse transcriptase PCR, SAH: subarachnoid hemorrhage, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, SDH: subdural hematoma, SE: status epilepticus, SIRPIDs: stimulus-induced rhythmic, periodic or ictal discharges, TIA: transient ischemic attack, VZV: varicella-zoster virus, WBC: white blood cells, WM: white matter.
Seizures and SE
Seizures and/or SE were recorded in 10 patients out of 111 included in the series of Pellinen et al. (2020), in 2 out of 22 in the series of Louis et al. (2020), in 1 out of 37 in the series of Ayub et al. (2020), in 1 out of 15 in the series of Pasini et al. (2020), in 1 out of 27 in the series of Scullen et al. (2020), and in an unknown precise number of patients out of the 73 included in the series of Chougar et al. (2020) (Table 1).
Seizures and/or SE were recorded in reports of patients without any acute or chronic cortical lesions on brain imaging nor cerebrospinal fluid (CSF) abnormalities. The EEG of the patient reported by Balloy et al. (2020) revealed two widespread, but predominantly in frontal localizations, seizures that were interrupted by a moderate interictal frontal activity. Sohal and Mansur (2020) reported a patient whose 24-h EEG revealed six left temporal seizures and left temporal sharp waves. One of the two patients reported by Somani et al. (2020) displayed, on a continuous EEG (cEEG) monitoring, multiple seizures emanating from the midline and left frontocentral regions (Figure 1B). Hepburn et al. (2020) reported the cases of two patients whose cEEG monitoring showed, for the first one, three focal seizures arising from the right frontocentral region and, for the second one, left more than right frontotemporal seizures which progressed to focal SE. The EEG of the patient reported by Le Guennec et al. (2020) revealed a non-convulsive SE (NCSE) over the right frontal region. The brain MRI of this patient only showed peri-ictal diffusion abnormalities over the right orbital and mesial prefrontal cortex and right caudate nucleus. Flamand et al. (2020) reported the case of a patient who benefited from several EEG. The first two EEG findings were consistent with a bilateral frontal SE. One EEG in the series of five patients reported by Chen et al. (2020) showed a bifrontal SE, and another one revealed a generalized NCSE. Finally, the EEG of the patient reported by Rodrigo-Armenteros et al. (2020) showed a bilateral frontotemporal NCSE.
Seizures and/or SE were recorded more rarely in patients with acute CNS lesions on brain imaging and/or significant CSF abnormalities, of either vascular or inflammatory origin. Among the four patients with a PRES reported by Parauda et al. (2020), two had seizures or SE emanating from posterior regions: for the first one, a focal NCSE arising from the left posterior quadrant and, for the second one, focal seizures arising from the right posterior quadrant. The history of a 2-month-old boy was published by Schupper et al. (2020). His brain imaging revealed multiple infarctions with hemorrhagic transformations, and his cEEG showed NCSE. Zanin et al. (2020) published the case of a patient with diffuse CNS demyelinating lesions on brain and spine imaging whose EEG revealed two seizures starting from the right frontotemporal region and diffusing in the homologous contralateral hemisphere. Hussein et al. (2020) reported the case of a patient with an ADEM whose EEG revealed left hemispheric seizures and, 3 days later, brief focal right posterior seizures. Finally, Bernard-Valnet et al. (2020) reported the history of a patient with a lymphocytic meningitis on CSF analysis with normal brain MRI whose EEG showed a focal anterior NCSE.
Seizures and/or SE were recorded in patients with a prior neurological history and radiological sequelae but without any acute lesions. The EEG of the second patient, who had a prior history of skull base surgery, reported by Somani et al. (2020) showed recurrent seizures emanating from either right or left frontocentroparietal regions. Vollono et al. (2020) reported the case of a left frontocentrotemporal SE in a patient with a remote herpes simplex virus 1 encephalitis.
Seizures were reported on cEEG in the series of 33 patients published by Radmard et al. (2020), as frontotemporal and parasagittal seizures in two patients, but without precise imaging or CSF results available for these two patients.
Rhythmic and Periodic Discharges
Rhythmic discharges were mentioned in series, as generalized rhythmic delta activity (GRDA) (Ayub et al., 2020; Galanopoulou et al., 2020; Louis et al., 2020; Pellinen et al., 2020; Petrescu et al., 2020), lateralized rhythmic delta activity (LRDA) (Ayub et al., 2020; Galanopoulou et al., 2020; Louis et al., 2020; Pellinen et al., 2020), and frontal intermittent rhythmic delta activity (Canham et al., 2020; Pasini et al., 2020) (Table 1). Rhythmic patterns were also reported in a few case reports. Vandervorst et al. (2020) published the EEG of a patient with a clinical and radiological picture of encephalitis with temporal bilateral more left than right imaging abnormalities. The EEG showed short-lasting left temporal LRDA. In the series of Beach et al. (2020), one patient, with a previous history of dementia with Lewy bodies and remote traumatic brain injury, displayed GRDA with sharp contouring and bifrontal predominance, without any acute lesions on brain imaging. The EEG of the three other patients reported in the series of Chen et al. (2020) previously mentioned revealed GRDA, with unremarkable CSF analysis for the three and no acute lesions on brain imaging for one of them (unavailable for the two others). One EEG recorded among the seven patients reported by Anand et al. (2020) showed GRDA in a patient with extensive leukoencephalopathy on brain MRI and normal CSF sample.
Periodic discharges were noted in series, as generalized periodic discharges (GPDs) (Ayub et al., 2020; Galanopoulou et al., 2020; Louis et al., 2020; Pellinen et al., 2020; Petrescu et al., 2020; Vespignani et al., 2020) and lateralized periodic discharges (LPDs) (Pellinen et al., 2020; Petrescu et al., 2020; Vespignani et al., 2020) (Table 1). Especially, in the series of Vespignani et al. (2020), five EEGs out of 26 showed periodic discharges. Four of these five patients were under mechanical ventilation (MV), and three were sedated. One patient suffered from a cardiac arrest. EEG showed periodic (with a < 4 s interval), monomorphic biphasic, delta activity, which was diffuse with frontal predominance for four and lateralized over right frontal area for one. The second patient reported in the work of Beach al. previously mentioned presented with a left-sided acute-on-chronic subdural hematoma (SDH) due to a fall with head trauma. The EEG showed frequent runs of epileptiform GPDs (Beach et al., 2020). Young et al. (2020) reported 1–1.5 Hz LPDs and diffuse delta–theta slowing in a patient who displayed Creutzfeldt–Jakob disease in tandem with symptomatic onset of COVID-19. Conte et al. (2020) published the history of a patient who presented a severe COVID-19 pneumonia and then a PRES-like encephalopathy. She displayed focal seizures, and after seizure treatment, EEG revealed LPDs in the right posterior regions. Vellieux et al. (2020) published the EEG of two critically ill patients who displayed a severe COVID-19 pneumonia requiring MV. For the first one, the brain MRI was consistent with a hypoxic encephalopathy, and the EEG was recorded while he was sedated and under extracorporeal membrane oxygenation. For the second one, the EEG was recorded 24 h after sedation discontinuation. EEG revealed continuous, symmetric, non-reactive, generalized but mainly bifrontal, monomorphic diphasic or even triphasic, periodic (with a short interval of 1–2 s) delta slow waves (Figure 1C). One patient, without any acute abnormalities on brain MRI and with normal CSF analysis, reported by Delorme et al. (2020) showed GPDs. In the previously mentioned case reported by Le Guennec et al. (2020), a control follow-up EEG was recorded the day after the first EEG. It showed persistent right frontal LPDs with a short interval (0.7–1.2 s). The brain MRI performed 1 month later was normal. Finally, the previously mentioned patient reported by Flamand et al. (2020) who benefited from iterative EEG showed, on the last two recordings, a generalized periodic triphasic activity with short periods (1–1.5 s) over a worsened background activity, without concomitant metabolic disorders.
Spectral Analysis
Two studies reported quantitative analysis of EEG (qEEG) in COVID-19 patients. The study of Pastor et al. (2020) reported 20 patients with COVID-19 encephalopathy for whom standard visual analysis of EEG showed scarce abnormalities. However, compared to 31 infectious toxic encephalopathy patients and 21 post-cardiorespiratory arrest encephalopathy patients, some qEEG features were specific in COVID-19 patients, such as the distribution of EEG bands, the structure of Shannon’s spectral entropy, and the hemispheric connectivity. Finally, the study of Pati et al. (2020) showed that some qEEG markers, especially an increase in both the theta power and its temporal variance during EEG reactivity, can predict a good neurological outcome in 10 critically ill COVID-19 patients.
Discussion
The vast majority of these studies emphasized the absence of specificity of EEG abnormalities reported in COVID-19 patients, as generalized slowing of the background activity, focal slowing sometimes associated with sharp waves, seizures, SE, and predictable pattern of metabolic/toxic or postanoxic encephalopathy in ICU patients. Numerous EEGs in the context of COVID-19 were recorded in elderly patients and mainly in male patients, with multiple comorbidities especially chronic brain disorders, under various psychotropic drugs or in critically ill conditions. Confounding factors such as infections, metabolic disturbances, severe hypoxemia, hyperthermia, and psychotropic drugs (such as antiepileptic or sedative drugs) were frequent at the time of EEG recordings. All these confounding factors may contribute to the modification of brain activity and therefore EEG findings. Thus, based on the current literature, it seems not possible to identify a specific EEG pattern due to the suspected neuroinvasion of SARS-CoV-2 in patients who displayed neurological manifestations of COVID-19.
Most current studies with available EEG data are case reports or retrospective single-center series. All reported patients are very heterogeneous concerning prior neurological histories, illness severity, and use of psychotropic drugs. Moreover, some studies reported EEG recorded with limited montage and number of electrodes that may limit the detection of EEG abnormalities. EEG is not a systematic exam in the diagnostic workup of COVID-19 patients. All patients reported in the current literature had an EEG for an urgent clinical indication due to concerning neurological symptoms. A wider neurological multimodality screening, including EEG, of COVID-19 patients may be suggested to grow the body of knowledge on the SARS-CoV-2 infection. However, it will face many logistic difficulties and ethical and safety concerns regarding the availability of trained personnel to EEG recordings and the risk of contamination with the SARS-CoV-2.
It should be pointed out that many EEG abnormalities reported were recorded over anterior or frontal regions. Regardless of EEG montage used by clinicians and neurophysiologists, it thus seems essential to include frontal electrodes. Periodicity, morphology, and reactivity of these frontal abnormalities were not mentioned in all studies. Moreover, a few reported periodic patterns, as GPDs (Ayub et al., 2020; Beach et al., 2020; Delorme et al., 2020; Louis et al., 2020; Pellinen et al., 2020; Petrescu et al., 2020), GPDs with bifrontal predominance (Galanopoulou et al., 2020; Vellieux et al., 2020; Vespignani et al., 2020), and LPDs (Conte et al., 2020; Le Guennec et al., 2020; Pellinen et al., 2020; Petrescu et al., 2020; Vespignani et al., 2020; Young et al., 2020). In particular, these frontal periodic discharges were monomorphic and displayed a short interval, and the absence of reactivity was noted (Vellieux et al., 2020; Vespignani et al., 2020). These frontal periodic discharges may indicate an acute neurological process linked to the brain SARS-CoV-2 infection. In COVID-19 patients, the combination of the frontal localization of these EEG discharges, the frequently reported anosmia (Yazdanpanah et al., 2020), the olfactory bulb abnormalities found on brain imaging (Lin et al., 2020), and the hypometabolism within the orbitofrontal cortex on functional brain imaging (Karimi-Galougahi et al., 2020) may support the hypothesis whereby SARS-CoV-2 could invade the brain through the olfactory pathway. Then, it could spread transneuronally to other related brain areas particularly to frontal lobes, especially the orbital prefrontal cortex, which are adjacent to olfactory structures (Huang J. et al., 2020).
Conclusion
In the context of the SARS-CoV-2 infection, increasing EEG results were published along with clinical reports describing various neurological symptoms in patients with COVID-19. Due to the suspected neuroinvasion of SARS-CoV-2, the major issue when interpreting EEG is to determine whether the observed abnormalities reflect this viral neuroinvasion, a severe encephalopathy with systemic and brain inflammation, hypoxemia and hyperthermia, and/or many confounding factors especially due to critical illness. At this time, no study had described specific EEG abnormalities of the SARS-CoV-2 infection. The majority of currently reported EEGs showed generalized slowing, focal slowing, epileptiform discharges with seizures, and SE. However, frontal discharges, for some periodic, may integrate in the olfactory hypothesis of the CNS invasion of SARS-CoV-2. It reinforces the need to accumulate precise neurophysiological observations of COVID-19 patients worldwide and to aggregate multimodality screening of these patients also with clinical, radiological, biological, and neuropathological data.
Author Contributions
GV collected the data and wrote the manuscript. RS, SV, PJ, and AR-T revised the manuscript. M-PO suggested and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., et al. (2020). Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 77:e202687. 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel D., Shen M. Y., Abid Z., Hennigan C., Boneparth A., Miller E. H., et al. (2020). Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology 95 745–748. 10.1212/WNL.0000000000010652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza-Abildúa M. J., Novo-Aparicio S., Moreno-Zabaleta R., Algarra-Lucas M. C., Rojo Moreno-Arcones B., Salvador-Maya M. Á, et al. (2020). Encephalopathy in severe SARS-CoV2 infection: inflammatory or infectious? Int. J. Infect. Dis. 98 398–400. 10.1016/j.ijid.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P., Al-Faraj A., Sader E., Dashkoff J., Abdennadher M., Murugesan R., et al. (2020). Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 112:107335. 10.1016/j.yebeh.2020.107335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriuta D., Roger P.-A., Thibault W., Toublanc B., Sauzay C., Castelain S., et al. (2020). COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J. Neurol. 11 1–2. 10.1007/s00415-020-09975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Sajed S. (2020). Seizures related to coronavirus disease (COVID-19): case series and literature review. Cureus 12:e9378. 10.7759/cureus.9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayub N., Cohen J., Jing J., Jain A., Tesh R., Mukerji S. S., et al. (2020). Clinical electroencephalography findings and considerations in hospitalized patients with coronavirus SARS-CoV-2. medRxiv [Preprint]. 10.1101/2020.07.13.20152207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy G., Leclair-Visonneau L., Péréon Y., Magot A., Peyre A., Mahé P.-J., et al. (2020). Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin. Neurophysiol. 131 2059–2061. 10.1016/j.clinph.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach S. R., Praschan N. C., Hogan C., Dotson S., Merideth F., Kontos N., et al. (2020). Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry 65 47–53. 10.1016/j.genhosppsych.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benameur K., Agarwal A., Auld S. C., Butters M. P., Webster A. S., Ozturk T., et al. (2020). Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 26 2016–2021. 10.3201/eid2609.202122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Valnet R., Pizzarotti B., Anichini A., Demars Y., Russo E., Schmidhauser M., et al. (2020). Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur. J. Neurol. 10.1111/ene.14298 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun G., Hak J.-F., Coze S., Kaphan E., Carvelli J., Girard N., et al. (2020). COVID-19-White matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol. Neuroimmunol. Neuroinflamm. 7:e777. 10.1212/NXI.0000000000000777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canham L. J. W., Staniaszek L. E., Mortimer A. M., Nouri L. F., Kane N. M. (2020). Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a case series. Clin Neurophysiol Pract. 5 199–205. 10.1016/j.cnp.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti G., Vabanesi M., Chieffo R., Fanelli G., Minicucci F., Agosta F., et al. (2020). Cerebral involvement in COVID-19 is associated with metabolic and coagulation derangements: an EEG study. J. Neurol. 10.1007/s00415-020-09958-2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont H., San-Galli A., Martino F., Couratier C., Joguet G., Carles M., et al. (2020). Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J. Neurol. 267 3121–3127. 10.1007/s00415-020-09986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Toprani S., Werbaneth K., Falco-Walter J. (2020). Status epilepticus and other EEG findings in patients with COVID-19: a case series. Seizure 81 198–200. 10.1016/j.seizure.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougar L., Shor N., Weiss N., Galanaud D., Leclercq D., Mathon B., et al. (2020). Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology 297:202422. 10.1148/radiol.2020202422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G., Avignone S., Carbonara M., Meneri M., Ortolano F., Cinnante C., et al. (2020). COVID-19-associated PRES-like encephalopathy with perivascular gadolinium enhancement. AJNR Am. J. Neuroradiol. 41 2206–2208. 10.3174/ajnr.A6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano P., Nencha U., De Stefano L., Mégevand P., Seeck M. (2020). Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin. Neurophysiol. Pract. 5 125–129. 10.1016/j.cnp.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L., Gollion C., Grouteau G., Rousset D., Jimena G., Roustan J., et al. (2020). COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J. Neurol. Neurosurg. Psychiatry 91 1004–1006. 10.1136/jnnp-2020-323678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C., Paccoud O., Kas A., Hesters A., Bombois S., Shambrook P., et al. (2020). Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur. J. Neurol. 27 2651–2657. 10.1111/ene.14478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Pérez C., Ramos C., López-Cruz A., Muñoz Olmedo J., Lázaro González J., De Vega-Ríos E., et al. (2020). Acutely altered mental status as the main clinical presentation of multiple strokes in critically ill patients with COVID-19. Neurol. Sci. 41 2681–2684. 10.1007/s10072-020-04679-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L., Coughlan C., Karunaratne K., Gorgoraptis N., Varley J., Husselbee J., et al. (2020). Immunosuppression for intracranial vasculitis associated with SARS-CoV-2: therapeutic implications for COVID-19 cerebrovascular pathology. J. Neurol. Neurosurg. Psychiatry 10.1136/jnnp-2020-324291 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Dugue R., Cay-Martínez K. C., Thakur K. T., Garcia J. A., Chauhan L. V., Williams S. H., et al. (2020). Neurologic manifestations in an infant with COVID-19. Neurology 94 1100–1102. 10.1212/WNL.0000000000009653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L., Xu P., Liu A. (2020). Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 87:33. 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.-M., et al. (2020). CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology 96 e294–e300. 10.1212/WNL.0000000000010977 [DOI] [PubMed] [Google Scholar]
- Ekstrand J. J., Herbener A., Rawlings J., Turney B., Ampofo K., Korgenski E. K., et al. (2010). Heightened neurologic complications in children with pandemic H1N1 influenza. Ann. Neurol. 68 762–766. 10.1002/ana.22184 [DOI] [PubMed] [Google Scholar]
- Elgamasy S., Kamel M. G., Ghozy S., Khalil A., Morra M. E., Islam S. M. S. (2020). First case of focal epilepsy associated with SARS-coronavirus-2. J. Med. Virol. 92 2238–2242. 10.1002/jmv.26113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M. A., Benjamin L., Singh B., Lant S., Michael B. D., Easton A., et al. (2020). Neurological associations of COVID-19. Lancet Neurol. 19 767–783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zein R. S., Cardinali S., Murphy C., Keeling T. (2020). COVID-19-associated meningoencephalitis treated with intravenous immunoglobulin. BMJ Case Rep. 13:e237364. 10.1136/bcr-2020-237364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Fadakar N., Akbari A., Lotfi M., Farazdaghi M., Javanmardi F., et al. (2020). Seizure in patients with COVID-19. Neurol. Sci. 10.1007/s10072-020-04731-9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa P. S., Rizvi Z., Sharma P., Hindi F., Filatov A. (2020). Neurological complications of coronavirus disease (COVID-19): encephalopathy, mri brain and cerebrospinal fluid findings: case 2. Cureus 12:e7930. 10.7759/cureus.7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian S., Glick L. R., Vogels C. B. F., Thomas J., Chiarella J., Casanovas-Massana A., et al. (2020). Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 20:248. 10.1186/s12883-020-01812-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley M., Zuberi J. (2020). COVID-19 precipitating status epilepticus in a pediatric patient. Am. J. Case Rep. 21:e925776. 10.12659/AJCR.925776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Cavallieri F., Canali E., Valzania F. (2020). First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol. Sci. 41 1651–1653. 10.1007/s10072-020-04460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P. S. (2020). Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 12:e7352. 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D., Threlkeld Z. D., Bodien Y. G., Kirsch J. E., Huang S. Y., Schaefer P. W., et al. (2020). Intact brain network function in an unresponsive patient with COVID -19. Ann. Neurol. 88 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand M., Perron A., Buron Y., Szurhaj W. (2020). Pay more attention to EEG in COVID-19 pandemic. Clin. Neurophysiol. 131 2062–2064. 10.1016/j.clinph.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freij B. J., Gebara B. M., Tariq R., Wang A.-M., Gibson J., El-Wiher N., et al. (2020). Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 20:429. 10.1186/s12887-020-02308-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A. S., Ferastraoaru V., Correa D. J., Cherian K., Duberstein S., Gursky J., et al. (2020). EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open 5 314–324. 10.1002/epi4.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Howard M., Herranz-Aguirre M., Moreno-Galarraga L., Urretavizcaya-Martínez M., Alegría-Echauri J., Gorría-Redondo N., et al. (2020). Case report: benign infantile seizures temporally associated with COVID-19. Front. Pediatr. 8:507. 10.3389/fped.2020.00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughan M., Connolly S., Direkze S., Kinsella J. A. (2020). Acute new-onset symptomatic seizures in the context of mild COVID-19 infection. J. Neurol. 10.1007/s00415-020-10214-w [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi S., Lagarde S., Harle J.-R., Boucraut J., Guedj E. (2020). Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from 18F-FDG PET imaging and neuronal autoantibodies. J. Nucl. Med. 61 1726–1729. 10.2967/jnumed.120.249292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M. V., Sehgal K., Nair N., Mahajan S., Sehrawat T. S., et al. (2020). Extrapulmonary manifestations of COVID-19. Nat. Med. 26 1017–1032. 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- Haddad S., Tayyar R., Risch L., Churchill G., Fares E., Choe M., et al. (2020). Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases 21:e00814. 10.1016/j.idcr.2020.e00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R., Nanda S., Prasad A., Anand R., Zutshi D., Dass S. K., et al. (2020). Covid-19-associated acute haemorrhagic leukoencephalomyelitis. Neurol. Sci. 10.1007/s10072-020-04703-z [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrogate S., Mortimer A., Burrows L., Fiddes B., Thomas I., Rice C. M. (2020). Non-aneurysmal subarachnoid haemorrhage in COVID-19. Neuroradiology 10.1007/s00234-020-02535-4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. (2020a). Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 382 2268–2270. 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Schenck M., Severac F., Clere-Jehl R., et al. (2020b). Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care 24:491. 10.1186/s13054-020-03200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., et al. (2020). Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit. Care 10.1007/s12028-020-01006-1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Eichelberger H., Chan M., Valdes E., Kister I., Krupp L., et al. (2020). Pearls and Oy-sters: leukoencephalopathy in critically ill COVID-19 patients. Neurology 95 753–757. 10.1212/WNL.0000000000010636 [DOI] [PubMed] [Google Scholar]
- Huang J., Zheng M., Tang X., Chen Y., Tong A., Zhou L. (2020). Potential of SARS-CoV-2 to cause CNS infection: biologic fundamental and clinical experience. Front. Neurol. 11:659. 10.3389/fneur.2020.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein O., Abd Elazim A., Torbey M. T. (2020). Covid-19 systemic infection exacerbates pre-existing acute disseminated encephalomyelitis (ADEM). J. Neuroimmunol. 349:577405. 10.1016/j.jneuroim.2020.577405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim F., Haddad N. (2014). New onset refractory status epilepticus in a young man with H1N1 infection. Case Rep. Neurol. Med. 2014:585428. 10.1155/2014/585428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K., Khatri A., Majure D. T. (2020). COVID-19 leading to acute encephalopathy in a patient with heart transplant. J. Heart Lung Transplant. 39 853–855. 10.1016/j.healun.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajani R., Apramian A., Vega A., Ubhayakar N., Xu P., Liu A. (2020). Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav. Immun. 88 28–29. 10.1016/j.bbi.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Galougahi M., Yousefi-Koma A., Bakhshayeshkaram M., Raad N., Haseli S. (2020). 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad. Radiol. 27 1042–1043. 10.1016/j.acra.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S., Stroud B., Parsons J., Schreiner T., Curtis D. J., Bagdure D., et al. (2011). Pediatric neurological complications of 2009 pandemic influenza A (H1N1). Arch. Neurol. 68 455–462. 10.1001/archneurol.2010.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo A., McLoughlin B., Cheema S., Weil R. S., Lambert C., Manji H., et al. (2020). Postinfectious brainstem encephalitis associated with SARS-CoV-2. J. Neurol. Neurosurg. Psychiatry 91 1013–1014. 10.1136/jnnp-2020-323816 [DOI] [PubMed] [Google Scholar]
- Kihira S., Delman B. N., Belani P., Stein L., Aggarwal A., Rigney B., et al. (2020). Imaging features of acute encephalopathy in patients with COVID-19: a case series. AJNR Am J Neuroradiol. 41 1804–1808. 10.3174/ajnr.A6715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos S., Tzortzakakis A., Kits A., Öhberg C., Kollia E., Ahoromazdae A., et al. (2020). Nervous System Involvement in COVID-19: results from a retrospective consecutive neuroimaging cohort. Radiology 297:202791. 10.1148/radiol.2020202791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guennec L., Devianne J., Jalin L., Cao A., Galanaud D., Navarro V., et al. (2020). Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia 61 e90–e94. 10.1111/epi.16612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Janaway B., Costello H., Trip A., Price G. (2020). Persistent psychotic symptoms following COVID-19 infection. BJPsych Open 6:e105. 10.1192/bjo.2020.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Lantos J. E., Strauss S. B., Phillips C. D., Campion T. R., Navi B. B., et al. (2020). Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am. J. Neuroradiol. 41 2001–2008. 10.3174/ajnr.A6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llansó L., Urra X. (2020). Posterior reversible encephalopathy syndrome in COVID-19 disease: a case-report. SN Compr. Clin. Med. 10.1007/s42399-020-00470-2 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente Ayuso L., Torres Rubio P., Beijinho do Rosário R. F., Giganto Arroyo M. L., Sierra-Hidalgo F. (2020). Bickerstaff encephalitis after COVID-19. J. Neurol. 10.1007/s00415-020-10201-1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logmin K., Karam M., Schichel T., Harmel J., Wojtecki L. (2020). Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J. Neurol. 267 2490–2491. 10.1007/s00415-020-09904-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S., Dhawan A., Newey C., Nair D., Jehi L., Hantus S., et al. (2020). Continuous electroencephalography characteristics and acute symptomatic seizures in COVID-19 patients. Clin. Neurophysiol. 131 2651–2656. 10.1016/j.clinph.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S., O’Kelly B., Woods S., Rowan C., Brady D., Sheehan G., et al. (2020). Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure 80 113–114. 10.1016/j.seizure.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganelli F., Vargas M., Iovino A., Iacovazzo C., Santoro L., Servillo G. (2020). Brainstem involvement and respiratory failure in COVID-19. Neurol. Sci. 41 1663–1665. 10.1007/s10072-020-04487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Guerrero A., Laespada-García M. I., Gómez-Grande A., Ruiz-Ortiz M., Blanco-Palmero V. A., Azcarate-Diaz F. J., et al. (2020). Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology 95 e2109–e2118. 10.1212/WNL.0000000000010282 [DOI] [PubMed] [Google Scholar]
- Morassi M., Bagatto D., Cobelli M., D’Agostini S., Gigli G. L., Bnà C., et al. (2020). Stroke in patients with SARS-CoV-2 infection: case series. J. Neurol. 267 2185–2192. 10.1007/s00415-020-09885-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guerra L., Provini F., Bordin G., et al. (2020a). COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J. Neuroimmunol. 349:577400. 10.1016/j.jneuroim.2020.577400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli L., Rondelli F., Ferri L., Rossini G., Cortelli P., Guarino M. (2020b). Subcortical myoclonus in COVID-19: comprehensive evaluation of a patient. Mov. Disord. Clin. Pract. 7 971–973. 10.1002/mdc3.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello A., Bassetti R., Radice A., Rossotti R., Puoti M., Corradin M., et al. (2020). Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav. Immun. 87 179–181. 10.1016/j.bbi.2020.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda P. K., Sharawat I. K., Panda P., Natarajan V., Bhakat R., Dawman L. (2020). Neurological Complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J. Trop. Pediatr. 10.1093/tropej/fmaa070 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parauda S. C., Gao V., Gewirtz A. N., Parikh N. S., Merkler A. E., Lantos J., et al. (2020). Posterior reversible encephalopathy syndrome in patients with COVID-19. J. Neurol. Sci. 416:117019. 10.1016/j.jns.2020.117019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. (2020). COVID-19-associated acute disseminated encephalomyelitis (ADEM). J. Neurol. 10.1007/s00415-020-09951-9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini E., Bisulli F., Volpi L., Minardi I., Tappatà M., Muccioli L., et al. (2020). EEG findings in COVID-19 related encephalopathy. Clin. Neurophysiol. 131 2265–2267. 10.1016/j.clinph.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor J., Vega-Zelaya L., Martín Abad E. (2020). Specific EEG encephalopathy pattern in SARS-CoV-2 patients. J. Clin. Med. 9:1545. 10.3390/jcm9051545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. W., Brown R. L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., et al. (2020). The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 143 3104–3120. 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S., Toth E., Chaitanya G. (2020). Quantitative EEG markers to prognosticate critically ill patients with COVID-19: a retrospective cohort study. Clin. Neurophysiol. 131 1824–1826. 10.1016/j.clinph.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen J., Carroll E., Friedman D., Boffa M., Dugan P., Friedman D. E., et al. (2020). Continuous EEG findings in patients with COVID-19 infection admitted to a New York academic hospital system. Epilepsia 61 2097–2105. 10.1111/epi.16667 [DOI] [PubMed] [Google Scholar]
- Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., et al. (2020). Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 28 248–258. 10.1111/ene.14491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu A.-M., Taussig D., Bouilleret V. (2020). Electroencephalogram (EEG) in COVID-19: a systematic retrospective study. Neurophysiol. Clin. 50 155–165. 10.1016/j.neucli.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Masciocchi S., Volonghi I., Crabbio M., Magni E., De Giuli V., et al. (2020a). Clinical presentation and outcomes of SARS-CoV-2 related encephalitis: the ENCOVID multicentre study. J. Infect. Dis. 28:jiaa609. 10.1093/infdis/jiaa609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., et al. (2020b). Steroid-responsive encephalitis in coronavirus disease 2019. Ann. Neurol. 88 423–427. 10.1002/ana.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princiotta Cariddi L., Tabaee Damavandi P., Carimati F., Banfi P., Clemenzi A., Marelli M., et al. (2020). Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J. Neurol. 10.1007/s00415-020-10001-7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin D., Vargas M.-I., Thieffry C., Schibler M., Grosgurin O., Pugin J., et al. (2020). COVID-19-related encephalopathy responsive to high doses glucocorticoids. Neurology 95 543–546. 10.1212/WNL.0000000000010354 [DOI] [PubMed] [Google Scholar]
- Rábano-Suárez P., Bermejo-Guerrero L., Méndez-Guerrero A., Parra-Serrano J., Toledo-Alfocea D., Sánchez-Tejerina D., et al. (2020). Generalized myoclonus in COVID-19. Neurology 95 e767–e772. 10.1212/WNL.0000000000009829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Ishida K. (2020). COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J. Neurol. Sci. 415:116945. 10.1016/j.jns.2020.116945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmard S., Epstein S. E., Roeder H. J., Michalak A. J., Shapiro S. D., Boehme A., et al. (2020). Inpatient neurology consultations during the onset of the SARS-CoV-2 New York city pandemic: a single center case series. Front. Neurol. 11:805. 10.3389/fneur.2020.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnis C., Qiu S., Jhaveri M., Da Silva I., Szewka A., Koffman L. (2020). Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J. Neurol. Sci. 418:117119. 10.1016/j.jns.2020.117119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C., Niculescu I., Patel S., Krishnan A. (2020). COVID-19 and involvement of the corpus callosum: potential effect of the cytokine storm? AJNR Am. J. Neuroradiol. 41 1625–1628. 10.3174/ajnr.A6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Armenteros P., Uterga-Valiente J. M., Zabala-Del-Arco J., Taramundi-Argüeso S., Erburu-Iriarte M., Antón-Méndez L., et al. (2020). Non-convulsive status epilepticus in a patient with COVID-19 infection. Clin. Neurophysiol. 131 2588–2590. 10.1016/j.clinph.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C. M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á, Layos-Romero A., García-García J., et al. (2020). Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95 e1060–e1070. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Gash F., De Mesmay M., Devys J.-M., Vespignani H., Blanc R., Engrand N. (2020). COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit. Care 24:419. 10.1186/s13054-020-03131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupper A. J., Yaeger K. A., Morgenstern P. F. (2020). Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv. Syst. 36 1579–1580. 10.1007/s00381-020-04755-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullen T., Keen J., Mathkour M., Dumont A. S., Kahn L. (2020). Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the new orleans experience. World Neurosurg. 141 e437–e446. 10.1016/j.wneu.2020.05.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N. K. (2020). EEG during the COVID-19 pandemic: what remains the same and what is different. Clin. Neurophysiol. 131:1462. 10.1016/j.clinph.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar R., Sheikh A. B., Suriya S. S., Upadhyay S., Zafar A. (2020). Neurological complications among native americans with COVID-19: our experience at a tertiary care academic hospital in the U.S. J. Stroke Cerebrovasc. Dis. 29:105260. 10.1016/j.jstrokecerebrovasdis.2020.105260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal S., Mansur M. (2020). COVID-19 Presenting with Seizures. IDCases 20:e00782. 10.1016/j.idcr.2020.e00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatelli M. D., do Amaral L. F., Veiga V. C., Rojas S. S. O., Omar S., Marussi V. H. R. (2020). Neurovascular and perfusion imaging findings in coronavirus disease 2019: case report and literature review. Neuroradiol. J. 33 368–373. 10.1177/1971400920941652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani S., Pati S., Gaston T., Chitlangia A., Agnihotri S. (2020). De novo status epilepticus in patients with COVID-19. Ann. Clin. Trans. Neurol. 7 1240–1244. 10.1002/acn3.51071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedung Wettervik T., Kumlien E., Rostami E., Howells T., von Seth M., Velickaite V., et al. (2020). Intracranial pressure dynamics and cerebral vasomotor reactivity in coronavirus disease 2019 patient with acute encephalitis. Crit. Care Explor. 2:e0197. 10.1097/CCE.0000000000000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T., Jason Q. W. M., Min Y. J., Wai K. H. S., Yuan M. Y., Yee J. C. C., et al. (2020). Encephalopathy in COVID-19 patients; viral, parainfectious, or both? eNeurologicalSci 21:100275. 10.1016/j.ensci.2020.100275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervorst F., Guldolf K., Peeters I., Vanderhasselt T., Michiels K., Berends K. J., et al. (2020). Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip. Neurosurg. 22:100821. 10.1016/j.inat.2020.100821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellieux G., Rouvel-Tallec A., Jaquet P., Grinea A., Sonneville R., d’Ortho M.-P. (2020). COVID-19 associated encephalopathy: is there a specific EEG pattern? Clin. Neurophysiol. 131 1928–1930. 10.1016/j.clinph.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespignani H., Colas D., Lavin B. S., Soufflet C., Maillard L., Pourcher V., et al. (2020). Report on electroencephalographic findings in critically ill patients with COVID -19. Ann. Neurol. 88 626–630. 10.1002/ana.25814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M. K., et al. (2020). Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 95 445–449. 10.1212/WNL.0000000000010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollono C., Rollo E., Romozzi M., Frisullo G., Servidei S., Borghetti A., et al. (2020). Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure 78 109–112. 10.1016/j.seizure.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah N., Saghazadeh A., Rezaei N. (2020). Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19). Rev. Neurosci. 10.1515/revneuro-2020-0039 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Young M. J., O’Hare M., Matiello M., Schmahmann J. D. (2020). Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav. Immun. 89 601–603. 10.1016/j.bbi.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid M. J., Baig A., Galvez-Jimenez N., Martinez N. (2020). Hemorrhagic stroke in setting of severe COVID-19 infection requiring Extracorporeal Membrane Oxygenation (ECMO). J. Stroke Cerebrovasc. Dis. 29:105016. 10.1016/j.jstrokecerebrovasdis.2020.105016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P. P., Renisi G., Signorini L., Migliorati K., et al. (2020). SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 162 1491–1494. 10.1007/s00701-020-04374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi A., Ramezani M., Roozbeh M., Darazam I. A., Sahraian M. A. (2020). A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult. Scler. Relat. Disord. 44:102324. 10.1016/j.msard.2020.102324 [DOI] [PMC free article] [PubMed] [Google Scholar]