Figure 5.
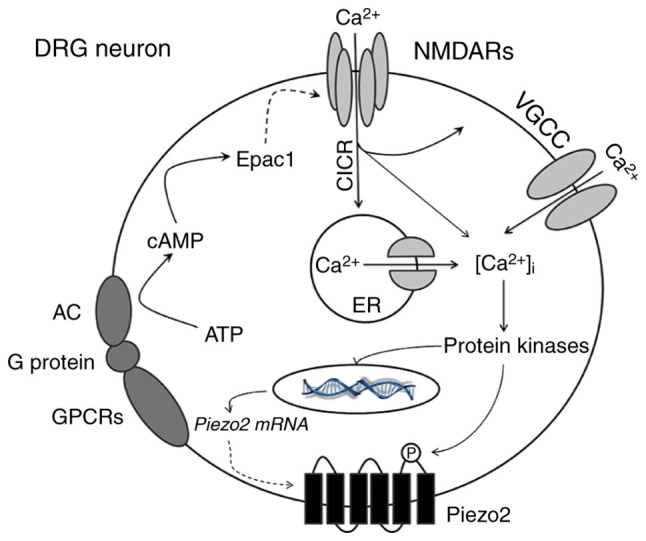
Schematic representation of NR2B-mediated Epac1-Piezo2 signaling pathway possibly participating in the mechanical allodynia of BCP. In the mouse model of BCP, peripheral nociceptive stimulation leads to the activation of GPCRs in DRG neurons which triggers the conversion of ATP into cAMP. As an important second messenger, cAMP directly activates Epac1 protein and then regulates the activity of NR2B-containing NMDA receptors. Activated NMDA receptors cause a significant increase in [Ca2+]i through multiple ways, such as the directly influx of calcium ions, CICR and VGCC. Various calcium-dependent protein kinases (such as ERK or CaMK) may be subsequently activated. On the one hand, the synthesis of Piezo2 mRNA or protein is increased; on the other hand, phosphorylation of Piezo2 enhances its function which mediate large amount of cations influx. Based on these mechanisms, the excitability of neurons continues to escalate and ultimately increases the transmission of peripheral nociceptive signals to the center, which leads to the production and maintenance of mechanical hyperalgesia in BCP. GPCRs, G protein-coupled receptors; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; DRG, dorsal root ganglion; CICR, calcium-induced calcium release; VGCC, voltage-gated calcium channel; ERK, extracellular-signal regulated kinase; CaMK, calmodulin-dependent kinase.
