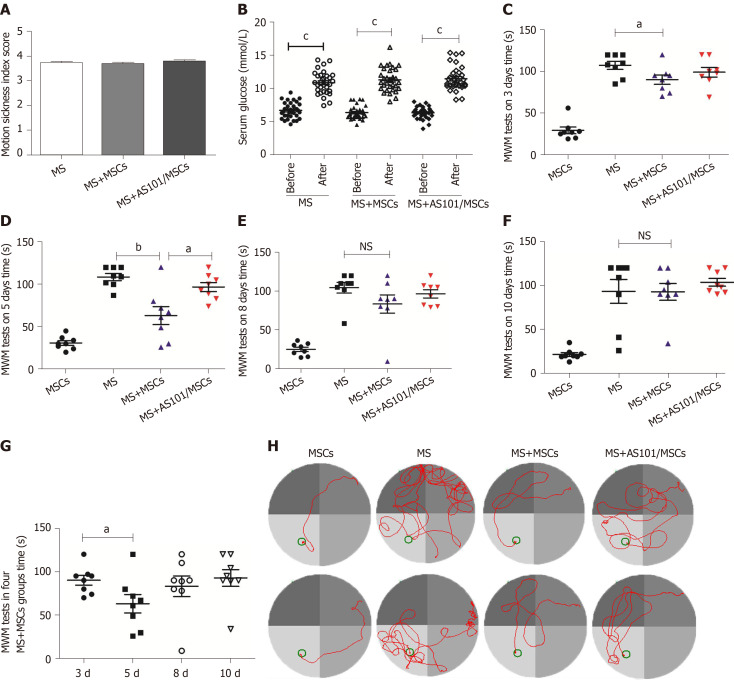
Figure 3.
Motion sickness model evaluation and effects of umbilical cord-derived mesenchymal stem cells treatment on performance of mice in Morris water maze tests. A: Motion sickness (MS) index score reflecting the degree of MS symptoms in mice; B: Serum glucose levels in the three groups before and after exposure to rotation (cP < 0.001); C-F: Time taken for the mice to reach the platform after different treatments. The time of the MS + mesenchymal stem cells (MS + MSCs) group was faster than that of the MS group at 3 d and 5 d (aP < 0.05; bP < 0.01); G: The 5-day group had the shortest time among the four MS + MSCs subgroups (aP < 0.05); H: Swimming trajectories in the MSCs, MS, MS + MSCs, and MS + AS101/MSCs groups on day 5; the platform is shown as a small circle. In addition to the MSCs group, the path of mice in the MS + MSCs group was clearer. MWM: Morris water maze; MS: Motion sickness; MSCs: Mesenchymal stem cells.