Abstract
Background:
Tumor progression following endocrine therapy is considered to indicate resistance to endocrine drugs due to a variety of mechanisms. An insufficient dose of endocrine drugs is one of the causes for treatment failure in some patients with high hormone-receptor (HR)-expressing advanced breast cancer. This study aimed to explore the efficacy of high-dose tamoxifen (TAM) treatment in patients with advanced breast cancer with highly expressed HR.
Materials & methods:
This was a single-arm, phase II pilot study that enrolled patients with advanced breast cancer with high HR expression (estrogen receptor ⩾60% and/or progesterone receptor ⩾60%) following routine endocrine therapy. All enrolled patients received a high-dose of TAM (100 mg/day) until disease progression. The primary endpoint was progression-free survival (PFS). The secondary endpoints included objective response rate (ORR), clinical benefit rate (CBR), overall survival (OS), and safety. Exploratory endpoints included the predictive value of 16α-18F-17β-fluoroestradiol quantitative positron emission tomography/computed tomography (18F-FES PET/CT) for treatment efficacy.
Results:
A total of 30 patients were enrolled between September 2017 and February 2019. The median PFS was 6 months [95% confidence interval (CI) 4.9–7.1] and the median OS was 15.6 months (95% CI 8.3–22.9). Five patients experienced a partial response (PR) and none experienced a complete response (CR), with an ORR of 16.7% and CBR of 33.3%. No severe adverse events were observed. Lesions with 18F-FES maximum standardized uptake value (SUVmax) ⩾4 had a significantly longer PFS [median 9.2 months, (95% CI 6.9–11.6)] compared with lesions with a 18F-FES SUVmax <4 [median 4.8 months, (95% CI 3.9–5.6); p = 0.022].
Conclusion:
A high-dose of TAM is effective and safe for patients with advanced breast cancer with high HR expression. 18F-FES SUVmax values may predict the local clinical benefits of high-dose TAM .
Trial Registration:
[ClinicalTrials.gov identifier: NCT0304565]
Keywords: breast cancer, hormone receptor-positive breast cancer, hormone therapy, PET, tamoxifen
Introduction
Approximately two-thirds of metastatic breast cancer (MBC) cases are hormone receptor (HR)-positive and candidates for endocrine therapy (ET) as the first treatment choice.1–4 Among various ET options, tamoxifen (TAM) is a classical selective estrogen receptor modulator (SERM) with a solid efficacy and safety record. Regardless of the level of HR expression, all patients commit to a standard administration dose of 20 mg/day; however, this dosage may be insufficient for those patients with high HR expression. The dose-dependent effect of TAM in TAM-resistant breast cancer cells has been reported in some in vitro studies. Low-dose TAM was found to function as an estrogen agonist, resulting in acquired resistance to TAM, while high-dose TAM inhibited TAM-resistant cell growth by blocking ERK1/2 and AKT activation.5 The addition of high-dose TAM could significantly inhibit the proliferation of breast cancer cells with high HR expression, whereas low-dose TAM treatment was ineffective.6,7 In addition, several clinical studies have indicated the potential efficacy of high-dose TAM for the treatment of MBC. A retrospective study reported that some patients with HR-positive MBC who failed to respond to the standard dose of TAM treatment experienced disease remission after increasing the dose of TAM to 80–90 mg/day.8 Another study enrolled 44 MBC patients who had progressed following the administration of conventional 20 mg/day TAM. It was found that 41 patients achieved significant improvement in symptoms when the TAM dose was increased to 100 mg/day.9 Based on the above evidence, it was hypothesized that for breast cancer with high HR expression, conventional 20 mg/day TAM may be not sufficient to completely block the proliferation-promoting effect of the HR, resulting in the failure of TAM therapy; however, these patients may benefit from a high dose of TAM.
In addition to ET regimens, effective and reliable predictors of the efficacy of ET are important for individualized treatment. Quantitative positron emission tomography/computed tomography (PET/CT) imaging of the endoplasmic reticulum (ER) using 16α-18F-17β-fluoroestradiol (18F-FES) can be used to accurately evaluate the heterogeneity of ER function in patients with metastatic disease.10 Prior studies have demonstrated that the level of baseline 18F-FES uptake can predict the response to hormonal therapy and may help guide treatment selection.11,12 This pilot clinical trial was conducted to explore the efficacy of high-dose TAM treatment in MBC patients with high HR expression and the predictive value of 18F-FES PET/CT on treatment efficacy.
Methods
Patients
This study was a single-arm pilot trial [ClinicalTrials.gov identifier: NCT03045653] conducted at the Sun Yat-sen Cancer Center in China from September 2017 to February 2019. This study was approved by the Ethics Committee for Clinical Investigation of the Sun Yat-sen Cancer Center. All subjects signed written informed consent. Eligible patients were females aged 18–70 years old with pathologically or histologically confirmed MBC and a high expression of ER and/or PR in metastatic or primary tumor lesions (immunohistochemical staining: ER-positive cells ⩾60% and/or PR-positive cells ⩾60%). The patients exhibited disease progression following adjuvant ET (a relapse or metastasis disease within 2 years after finishing adjuvant ET) or standard endocrine salvage treatment. Other eligibility criteria consisted of an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; life expectancy >3 months; and one or more measurable or non-measurable lesions. The key exclusion criteria included: ECOG ⩾2; receiving any other anti-tumor treatments; lactation or pregnancy; or a severe comorbidity. For patients who planned to undergo a baseline 18F-FES PET/CT examination, discontinuation of TAM for ⩾2 months was required to avoid false-negative 18F-FES results.
Interventions
Eligible patients were treated with 100 mg/day TAM until disease progression as indicated by radiological or clinical assessment, intolerable toxicity, or withdrawal of consent. For patients with HER-2 positive breast cancer, anti-HER-2 targeted therapies were allowed to be used.
Outcome measurements
The primary endpoint was progression-free survival (PFS), which was defined as the time from enrollment to the first occurrence of disease progression. The key secondary endpoints included the objective response rate [ORR, proportion of patients with a complete response (CR) or partial response (PR) per RECIST version 1.1], clinical benefit rate [CBR, the proportion of patients with CR, PR or stable disease (SD) ⩾6 months], overall survival (OS, the time from enrollment to death from any cause), safety, and tolerability. The exploratory endpoints included the predictive value of the 18F-FES maximum standardized uptake value (SUVmax) of 18F-FES PET/CT on the efficacy of high-dose TAM therapy. Assessments of treatment efficacy were performed every 3 months after starting the administration of 100 mg/day TAM. Follow up was conducted from the time of enrollment to disease progression or death.
The response evaluation criteria for a single lesion was defined based on the RECIST 1.1 standard: progressive disease (PD), the maximum diameter of a single metastasis lesion that was increased by more than 20%; CR: disappearance of target lesions; PR: the maximum diameter of the lesion decreased by more than 30%; and the remainder were considered to be stable disease (SD). The clinical benefit of a single lesion was defined according to the response evaluation criteria for a single lesion, as CR, PR, or SD ⩾6 months.
18F-FES and 18F-FDG PET/CT procedure
18F-FES was prepared as described previously.13 PET/CT imaging was performed from the skull base to the upper thigh using a Discovery PET/CT 690 or 710 scanner (GE Healthcare), 80–100 min after an intravenous injection of 111–222 MBq (3–6 mCi) of 18F-FES.14 PET/CT images were reconstructed using a manufacturer-provided iterative algorithm with four iterations and 18 subsets.18F-FDG (18F-fluorodeoxyglucose) PET/CT images were obtained from the skull base to the upper thigh using one of several different PET/CT scanners (Biograph Sensation 16 or Biograph TruePoint 40, Siemens Healthineers; or Discovery PET/CT 690, 690 Elite, or 710, GE Healthcare), 50 min–70 min after an intravenous injection of 5.2 MBq/kg–7.4 MBq/kg (0.14 mCi/kg–0.2 mCi/kg) of 18F-FDG as described previously.15 The reconstructed images were displayed on coronal, horizontal, sagittal, and three-dimensional (3D) volumetric films and reviewed independently by two nuclear medicine physicians. SUV was calculated automatically (SUV = average radioactivity per gram of tissue/radioactivity of the injected nuclides/mass). Regions of interest (ROIs) were drawn automatically by the Siemens MMWP workstation. To reduce the partial volume effect, the SUVmax corresponded to all pixels in an ROI, and the 18F-FES SUVmax of the corresponding lesions was measured.
Statistical analysis
The primary endpoint of PFS was determined based on an intention-to-treat set. If no PFS event was observed before the cut-off date, the last tumor assessment date was defined as censored. Safety was analyzed based on a safety analysis set defined as all patients who received at least one high-dose of TAM and had at least one post-treatment safety assessment. The median PFS was estimated using the Kaplan-Meier method and compared by a log-rank test. The comparison of continuous variables between two samples with a normal distribution was performed using a t-test. A comparison of the two categorical data was conducted using a Chi-square test. For all analyses, a two-tailed p ⩽ 0.05 was considered to be statistically significant, and all confidence intervals used a 95% confidence level. Statistical analyses were performed by the investigators at the Cancer Center of Sun Yat-sen University using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
A total of 30 patients were enrolled in the present study from September 2017 to February 2019. All patients were available for the intent-to-treat analysis. The data cut-off was 28 May 2020, with a median follow up of 12.7 months (range 5.6–18.8). At the end of the follow-up period, 25 patients died, all patients experienced disease progression and had a PFS event. No treatment discontinuation due to the intolerance of adverse events (AEs) was observed.
The median age of the overall subjects was 45 years (range 27–68 years). All enrolled patients received previous endocrine salvage therapy. There was a median of 3 (range 2–7) rounds of salvage therapy. The detailed baseline characteristics are described in Table 1.
Table 1.
Baseline characteristics.
| Characteristic | Number (range) | Percentage (%) |
|---|---|---|
| Median age (years) | 45 (27–68) | |
| Menopausal status | ||
| Menopausal | 5 | 17 |
| Postmenopausal | 25 | 83 |
| Pathological type | ||
| ILC | 3 | 10 |
| IDC | 27 | 90 |
| Expression of hormone receptor | ||
| ER, PR positive | 23 | 77 |
| ER positive, PR negative | 7 | 23 |
| Expression of HER2 | ||
| HER2 positive | 2 | 7 |
| HER2 negative | 28 | 93 |
| Median disease-free survival interval (months) | 36.5 (5.3–371.3) | |
| Advanced stage at first diagnosis | 6 | 20 |
| Median number of metastasis sites | 2 (1–4) | |
| Median number of therapeutic lines | 3 (2–7) | |
| Median treatment lines of ET | 2 (1–4) | |
| Median treatment lines of chemotherapy | 1 (0–3) | |
| PFS of previous treatment | ||
| Median PFS of first-line therapy (months) | 6 (2–60) | |
| Median PFS of second-line therapy (months) | 3 (1–24) | |
| Prior endocrine therapy | 30 | 100 |
| Prior TAM therapy | 18 | 60 |
| Adjuvant therapy | 13 | 43 |
| Median number of additional endocrine treatment lines | 2 (1–4) | |
| Median duration of time from stopping standard dose TAM to HD-TAM (months) | 22 (4–46) | |
| Salvage therapy for metastasis disease | 5 | 17 |
| Median number of additional endocrine treatment lines | 1 (1–3) | |
| Median duration of time from stopping standard dose TAM to HD-TAM (months) | 3 (1–6) | |
| Prior AIs therapy | 30 | 100 |
| Neo-adjuvant/adjuvant therapy | 22 | 73 |
| Salvage therapy for metastasis disease | 28 | 93 |
| Prior fulvestrant therapy | 11 | 37 |
| Previously received chemotherapy | 30 | 100 |
AIs, aromatase inhibitors; ER, estrogen receptor; ET, endocrine treatment; HD-TAM, high-dose tamoxifen; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PFS, progression-free survival; TAM, tamoxifen.
Treatment outcome
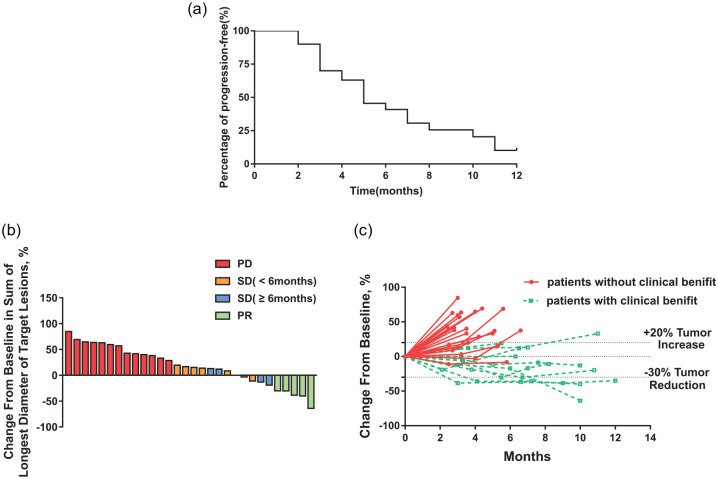
At the cut-off date of 28 May 2020 for final analysis, the median PFS was 6 months (95% CI 4.9–7.1) (Figure 1a). The median OS was 15.6 months (95% CI 8.3–22.9). The ORR in the ITT population was 16.7% and the CBR was 33.3%. No CR was observed and five patients exhibited PR. Five patients experienced SD for ⩾6 months, and seven patients had SD <6 months. A total of 13 cases of PD were reported. All patients had measurable lesions and nine patients (30%) experienced tumor regression and a change in the tumor size (Figure 1b,c). The median time to response was 3.47 months (95% CI 2.78–4.16) and the median duration of the response was 7.58 months (95% CI 5.92–9.24).
Figure 1.
Treatment outcomes. (a) Percentage of PFS. (b) Change from baseline in diameter of target lesions. (c) Change of tumor size over time.
PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Notably, three patients in our study had previously received the standard dose of 20 mg/day TAM as a rescue treatment and subsequently exhibited disease progression. However, all patients achieved clinical benefits (effective PR or SD ⩾6 months) after receiving high-dose 100 mg/day TAM treatment.
Qualitative 18F-FES PET/CT results
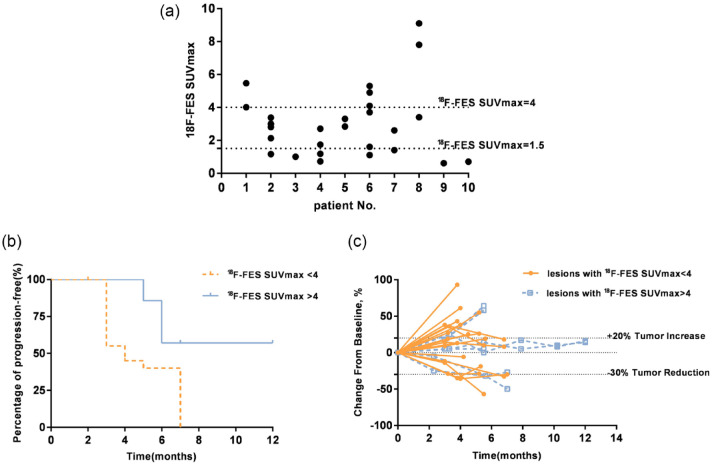
Out of the 30 patients, 10 received 18F-FES PET/CT at baseline with a total of 28 evaluable target metastatic lesions. The observed average value of 18F-FES SUVmax for all lesions was 3.03 (95% CI 2.22–3.83). According to previous studies,16,17 we defined a cut-off SUV of 1.5 to classify the 18F-FES-positive and 18F-FES-negative results. Out of the 10 patients, seven (70%) had at least one lesion with an 18F-FES SUVmax ⩾1.5. Out of the 28 target lesions, 20 (71.4%) had an 18F-FES SUVmax ⩾1.5 (Figure 2a). A discrete analysis of the 18F-FES SUVmax in each patient revealed that the minimum standard deviation of the 18F-FES SUVmax was 0.32, and the maximum standard deviation was 2.98. The lesions with clinical benefit (PR or SD ⩾6 months for a single lesion) had a significantly higher average 18F-FES SUVmax value [4.08 (95% CI 2.67–5.48)] compared with those without clinical benefit [2.10 (95% CI 2.40–3.7), p = 0.048].
Figure 2.
(a) Patient baseline 18F-FES SUVmax values, (b) Percentage of PFS, and (c) Change of tumor size over time.
18F-FES, 16α-18F-17β-fluoroestradiol; PFS, progression-free survival; SUVmax, maximum standardized uptake value.
Based on previous research,18 we defined a cut-off SUVmax of 4 to explore the predictive value of 18F-FES uptake by tumors for the response to high-dose TAM therapy. The logistic regression analysis showed that 18F-FES SUVmax ⩾4 was an independent factor for the clinical benefit of lesions (PR or SD ⩾6 months), and the OR was 2.01 (95% CI 1.07–3.78; p = 0.028). Kaplan–Meier analysis showed that lesions with an 18F-FES SUVmax ⩾4 had a significantly longer median progression-free period compared with those with an 18F-FES SUVmax <4 [9.2 months (95% CI 6.9–11.6) versus 4.8 months (95% CI 3.9–5.6); p = 0.022] (Figure 2b,c).
Representative case of treatment failure
Subject no. 11 was a 38-year-old woman who was diagnosed with stage IV invasive ductal carcinoma with metastases in the liver, lymph nodes, and bone. The patient experienced disease progression after receiving standard MBC therapies, including exemestane, goserellin, zoledronic acid, fulvestrant, and capecitabine. Before starting high-dose TAM treatment, a baseline 18F-FES PET/CT scan was conducted. The baseline immunohistochemistry of the biopsied liver lesions revealed the status of ER (80%), PR (10%), C-erbB-2 (0), and Ki-67 (20%). High-dose TAM treatment was initiated from September 2017, and the disease progressed in April 2018. A rebiopsy of the liver lesions in April 2018 revealed the change in the status of ER (0), PR (0), and C-erbB-2 (+). In the 18F-FES PET/CT scan after disease progression, the 18F-FES SUVmax of each lesion had decreased significantly, with a median decrease of 2.31 (95% CI 0.59–4.03; p = 0.018) (Supplemental Table S1; Figure 3).
Figure 3.
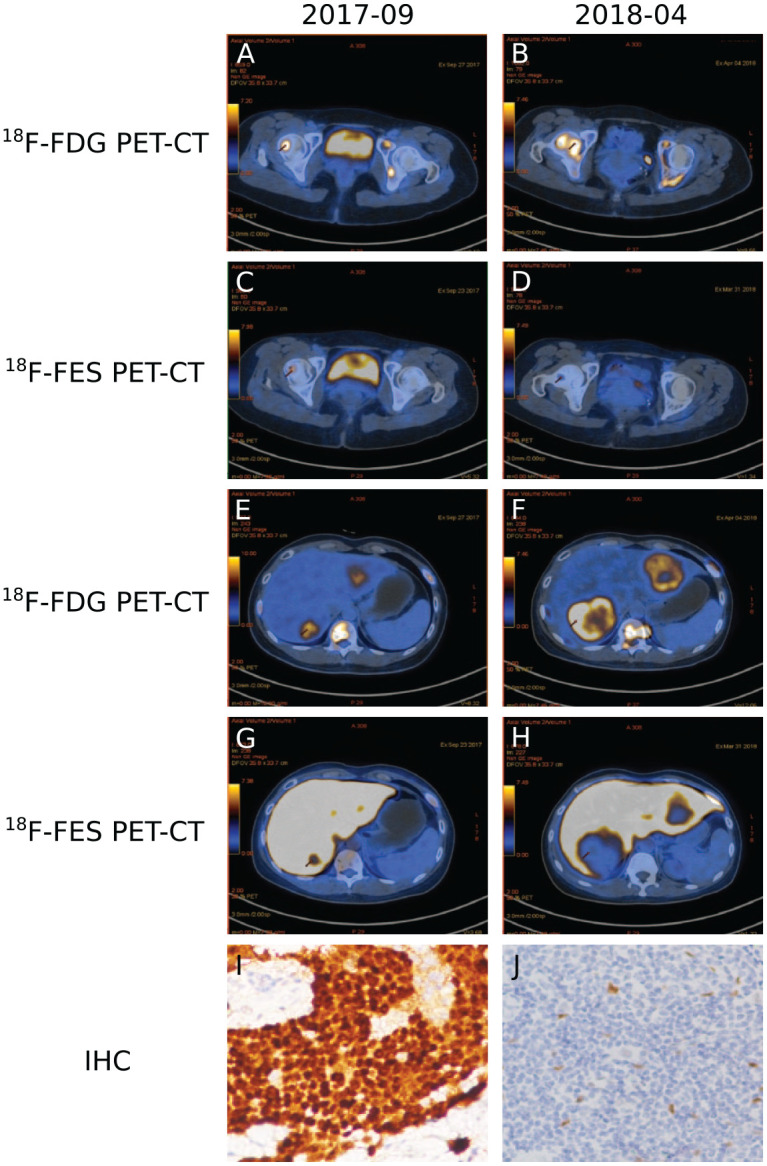
Quantitative PET/CT and IHC of biopsied liver lesions from patient 11. a, b 18F-FDG PET-CT. c, d 18F-FES PET-CT. e, f 18F-FDG PET-CT. g, h 18F-FES PET-CT. i, j IHC. a, c, e, g, i September 2017; b, d, f, h, j April 2018. Arrows indicate tumor lesions.
18F-FDG, 18F-fluorodeoxyglucose; 18F-FES, 16α-18F-17β-fluoroestradiol; IHC, immunohistochemistry; PET-CT, positron emission tomography/computed tomography.
Safety
Among the 30 enrolled patients, 14 experienced grade 1 or 2 AEs, including fatigue (seven cases), joint pain (five cases), hot flashes (four cases), insomnia (three cases), nausea (one case), and abdominal pain (one case) (Table 2). No grade ⩾3 or serious AEs (e.g., endometrial carcinoma) were observed. No AEs leading to treatment discontinuation or death were observed.
Table 2.
Adverse effects.
| Adverse effects | Grade (n) | |
|---|---|---|
| 1 | 2 | |
| Fatigue | 4 | 3 |
| Arthralgia | 4 | 1 |
| Flush | 3 | 1 |
| Insomnia | 3 | |
| Nausea | 1 | |
| Abdominal pain | 1 | |
Discussion
To our knowledge, this is the first prospective study to evaluate the efficacy of high-dose TAM treatment in patients with advanced breast cancer expressing high levels of HR. The results of this pilot trial indicate that for patients with high HR-expressing MBC, treatment with 100 mg/day TAM is effective and well-tolerated, with a median PFS of 6 months (95% CI 4.9–7.1). Moreover, a durable clinical benefit (PR or SD ⩾6 months) was reported for one-third of the patients. Notably, the majority of the patients in the trial progressed under treatment with standard ET, including TAM, AIs, and fulvestrant.
Few small-sample retrospective studies or case reports have explored the efficacy of high-dose ET for HR-positive advanced breast cancer. However, these studies provide limited reference value due to the vast differences in treatment options, as well as a lack of baseline information and modern examination techniques. In a phase II clinical trial, high-dose toremifene (120 mg/day) was administered as a first-line treatment for 23 MBC patients who relapsed after adjuvant therapy with aromatase inhibitors (AIs). These patients exhibited an improvement in the ORR of 13.0% (3/23) and CBR of 78.3% (18/23).19 Moreover, in a phase I clinical trial involving 41 patients with MBC, the patients were treated with oral Z-endoxifen at doses ranging from 20 mg/day to 160 mg/day. The previous salvage ET included AIs (n = 36), Fluvastatin (n = 21), and TAM (n = 15). The overall CBR was 26.3% (95% CI 13.4%–43.1%). Three of the patients who progressed after standard TAM treatment reported clinical benefits (PR or SD >6 months) after receiving high-dose oral endoxifen.20 The results of our trial are consistent with those of previous similar studies, and most treatment-related AEs were low grade and manageable. Notably, three patients in our study had previously received a standard dose of TAM therapy as a rescue treatment, and subsequently exhibited disease progression. However, all patients achieved a clinical benefit (effective PR or SD ⩾6 months) after receiving high-dose TAM treatment. For these patients, the previous conventional dose of TAM therapy may be too low to have a complete blocking effect on the ER receptors, and the high-dose TAM treatment has a higher efficacy with a dose-dependent effect. These findings also supported our hypothesis that progression following standard endocrine therapy for patients with advanced breast cancer expressing high levels of HR could be due partially to an insufficient dose of the administered endocrine drug, which was insufficient to completely suppress the HR.
In recent decades, the combined use of immune-targeted inhibitors, such as mTOR and CDK4/6 blockades, has significantly improved the outcomes of patients with HR positive MBC.21 The question of whether HD-TAM affects the activation of mTOR or CDK4/6 pathways remains attractive and unsolved. In MCF-7 breast cancer cell lines, the inhibition of mTOR activity can overcome TAM resistance and restores TAM response.22 In a phase II clinical trial exploring the combined use of Everolimus and TAM compared with TAM in patients with HR positive MBC, an improved clinical benefit rate and prolonged time to progression were observed.23 On the other hand, as a main target which blocks the transition from the G1 to the S phase of the cell cycle, CDK4/6 exhibits survival beneficial in both preclinical models and clinical trials for HR-positive breast cancer and exerts synergetic effects when combined with TAM treatment.24 Therefore, to explore the combination of HD-TAM and novel targeted therapy may indicate a potential effective treatment alternative in clinical practice.
Compared with a biopsy, which assesses only a specific region of a given tissue, whole-body 18F-FES PET/CT enables the ER expression in all metastases to be quantified. Some studies indicate that the baseline 18F-FES uptake in metastases can predict the efficacy of ET in patients with ER-positive tumors; however, the threshold of the 18F-FES SUVmax for selecting ET-responsive lesions remains controversial.25 In this study, tumor lesions with an 18F-FES SUVmax >4 had a median PFS of 9.2 months following multiline salvage ET, indicating a considerable clinical benefit. Our results suggest the potential utility of 18F-FES PET/CT as a predictive tool in conducting targeted therapies and personalized treatment management. However, due to the small sample size, our data were insufficient for determining whether the baseline 18F-FES SUVmax could predict the overall PFS for patients. Therefore, this issue should be further validated in a larger patient population.
A case report in this study provides clues that can be used to explore the potential reasons for high-dose TAM treatment failure. Prior to receiving high-dose TAM therapy, the results of the tumor biopsy and 18F-FES PET/CT confirmed multiple lesions with highly expressed ER. After disease progression, the 18F-FES PET/CT showed that the 18F-FES SUVmax of all progressive lesions was reduced significantly. The tumor biopsy also confirmed that the high level of ER expression had decreased substantially. At the 18F-FES PET/CT examination, the high-dose TAM had been discontinued for nearly 3 months. Since TAM and its metabolites had been cleared almost completely, its occupying effect was rare. Therefore, these findings suggest that the change in tumor clones and HR downregulation may be the reasons for the failure of high-dose TAM treatment.
The results of our study should be reviewed carefully in the context of several limitations. First, the small sample size of our trial limits the generalizability of our findings. Thus, larger studies are required to verify the efficacy of high-dose TAM treatment and the predictive value of 18F-FES PET/CT. Second, the lack of a control group has weakened the power. Third, since there were only two patients with HER-2 positive status in this trial, it is difficult to evaluate the effects of HER-2 status on HD-TAM treatment, which remains to be explored by further study. Fourth, although effective clinical benefit was observed, the molecular mechanisms of the high-dose TAM treatment remain unexplored. Moreover, whether the observed efficacy is related to dose-response effects or other specific antitumor pathways is still unknown.
Conclusion
The findings of the present study indicate that 100 mg/day TAM is an effective and safe treatment for patients with high HR-expressing MBC and subsequent progression after standard salvage ET. Moreover, the 18F-FES SUVmax may predict the clinical benefits of treatment with high-dose TAM therapy.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_1758835921993436 for High-dose tamoxifen in high-hormone-receptor-expressing advanced breast cancer patients: a phase II pilot study by Yanhong Su, Yarui Zhang, Xin Hua, Jiajia Huang, Xiwen Bi, Wen Xia, Xinyue Wang, Zhangzan Huang, Chenge Song, Yongyi Zhong, Yanxia Shi, Shusen Wang, South China Breast Cancer Group (SCBCG), Wei Fan and Zhongyu Yuan in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank the patients and their families who participated in the trial. We would also like to acknowledge the technical support of Nanfang Hospital.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sun Yat-sen University Cancer Center and Nanfang hospital. The funding body had no influence on the study design and execution, endpoint assessments, data collection, statistical analysis, or manuscript preparation in this trial.
Disclosure: All authors contributed to the writing and critical review of the manuscript. All authors made the decision to submit the manuscript for publication and declare no competing interests. The corresponding authors had full access to all the data.
ORCID iD: Shusen Wang  https://orcid.org/0000-0003-0139-5780
https://orcid.org/0000-0003-0139-5780
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yanhong Su, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Yarui Zhang, Department of Nuclear Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, China.
Xin Hua, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Jiajia Huang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Xiwen Bi, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Wen Xia, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Xinyue Wang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Zhangzan Huang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Chenge Song, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Yongyi Zhong, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Yanxia Shi, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Shusen Wang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, China.
Wei Fan, Department of Nuclear Medicine, Sun Yat-Sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510000, China.
Zhongyu Yuan, Department of Medical Oncology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng East Road, Guangzhou 510000, China.
References
- 1. DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017; 67: 439–448. [DOI] [PubMed] [Google Scholar]
- 2. Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet 2017; 389: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalmau E, Armengol-Alonso A, Munoz M, et al. Current status of hormone therapy in patients with hormone receptor positive (HR+) advanced breast cancer. Breast 2014; 23: 710–720. [DOI] [PubMed] [Google Scholar]
- 4. Harbeck N, Gnant M. Breast cancer. Lancet 2017; 389: 1134–1150. [DOI] [PubMed] [Google Scholar]
- 5. Wang LJ, Han SX, Bai E, et al. Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncol Rep 2013; 29: 1563–1569. [DOI] [PubMed] [Google Scholar]
- 6. Kodama F, Greene GL, Salmon SE. Relation of estrogen receptor expression to clonal growth and antiestrogen effects on human breast cancer cells. Cancer Res 1985; 45: 2720–2724. [PubMed] [Google Scholar]
- 7. Webster NJ, Green S, Jin JR, et al. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell 1988; 54: 199–207. [DOI] [PubMed] [Google Scholar]
- 8. Watkins SM. The value of high dose tamoxifen in postmenopausal breast cancer patients progressing on standard doses: a pilot study. Br J Cancer 1988; 57: 320–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stathopoulos GP, Trafalis D. High-dose tamoxifen in breast cancer bone metastasis. J BUON 2013; 18: 532–534. [PubMed] [Google Scholar]
- 10. Van Kruchten M, De Vries EGE, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 2013; 14: e465–e475. [DOI] [PubMed] [Google Scholar]
- 11. Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol 2006; 24: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 12. Dehdashti F, Flanagan FL, Mortimer JE, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med 1999; 26: 51–56. [DOI] [PubMed] [Google Scholar]
- 13. Mori T, Kasamatsu S, Mosdzianowski C, et al. Automatic synthesis of 16 alpha-[(18)F]fluoro-17beta-estradiol using a cassette-type [(18)F]fluorodeoxyglucose synthesizer. Nucl Med Biol 2006; 33: 281–286. [DOI] [PubMed] [Google Scholar]
- 14. Oh SJ, Chi DY, Mosdzianowski C, et al. The automatic production of 16alpha-[18F]fluoroestradiol using a conventional [18F]FDG module with a disposable cassette system. Appl Radiat Isot 2007; 65: 676–681. [DOI] [PubMed] [Google Scholar]
- 15. Seo MJ, Lee JJ, Kim HO, et al. Detection of internal mammary lymph node metastasis with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging 2014; 41: 438–445. [DOI] [PubMed] [Google Scholar]
- 16. Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med 2008; 49: 367–374. [DOI] [PubMed] [Google Scholar]
- 17. Van Kruchten M, Glaudemans AW, De Vries EF, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med 2012; 53: 182–190. [DOI] [PubMed] [Google Scholar]
- 18. Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 2001; 19: 2797–2803. [DOI] [PubMed] [Google Scholar]
- 19. Ogata H, Okamoto Y, Arima Y, et al. Phase II clinical trial of high-dose toremifene as primary hormone therapy in aromatase inhibitor-resistant breast cancer. Gan To Kagaku Ryoho 2013; 40: 749–753. [PubMed] [Google Scholar]
- 20. Goetz MP, Suman VJ, Reid JM, et al. First-in-human phase I study of the tamoxifen metabolite Z-endoxifen in women with endocrine-refractory metastatic breast cancer. J Clin Oncol 2017; 35: 3391–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2− metastatic breast cancer: biological mechanisms and new treatments. Cancers (Basel) 2019; 11: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res 2004; 10: 8059–8067. [DOI] [PubMed] [Google Scholar]
- 23. Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012; 30: 2718–2724. [DOI] [PubMed] [Google Scholar]
- 24. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25-35. [DOI] [PubMed] [Google Scholar]
- 25. Liao GJ, Clark AS, Schubert EK, et al. 18F-fluoroestradiol PET: current status and potential future clinical applications. J Nucl Med 2016; 57: 1269–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_1758835921993436 for High-dose tamoxifen in high-hormone-receptor-expressing advanced breast cancer patients: a phase II pilot study by Yanhong Su, Yarui Zhang, Xin Hua, Jiajia Huang, Xiwen Bi, Wen Xia, Xinyue Wang, Zhangzan Huang, Chenge Song, Yongyi Zhong, Yanxia Shi, Shusen Wang, South China Breast Cancer Group (SCBCG), Wei Fan and Zhongyu Yuan in Therapeutic Advances in Medical Oncology