Figure 1. Enrollment, Randomization, and Treatment Assignment.
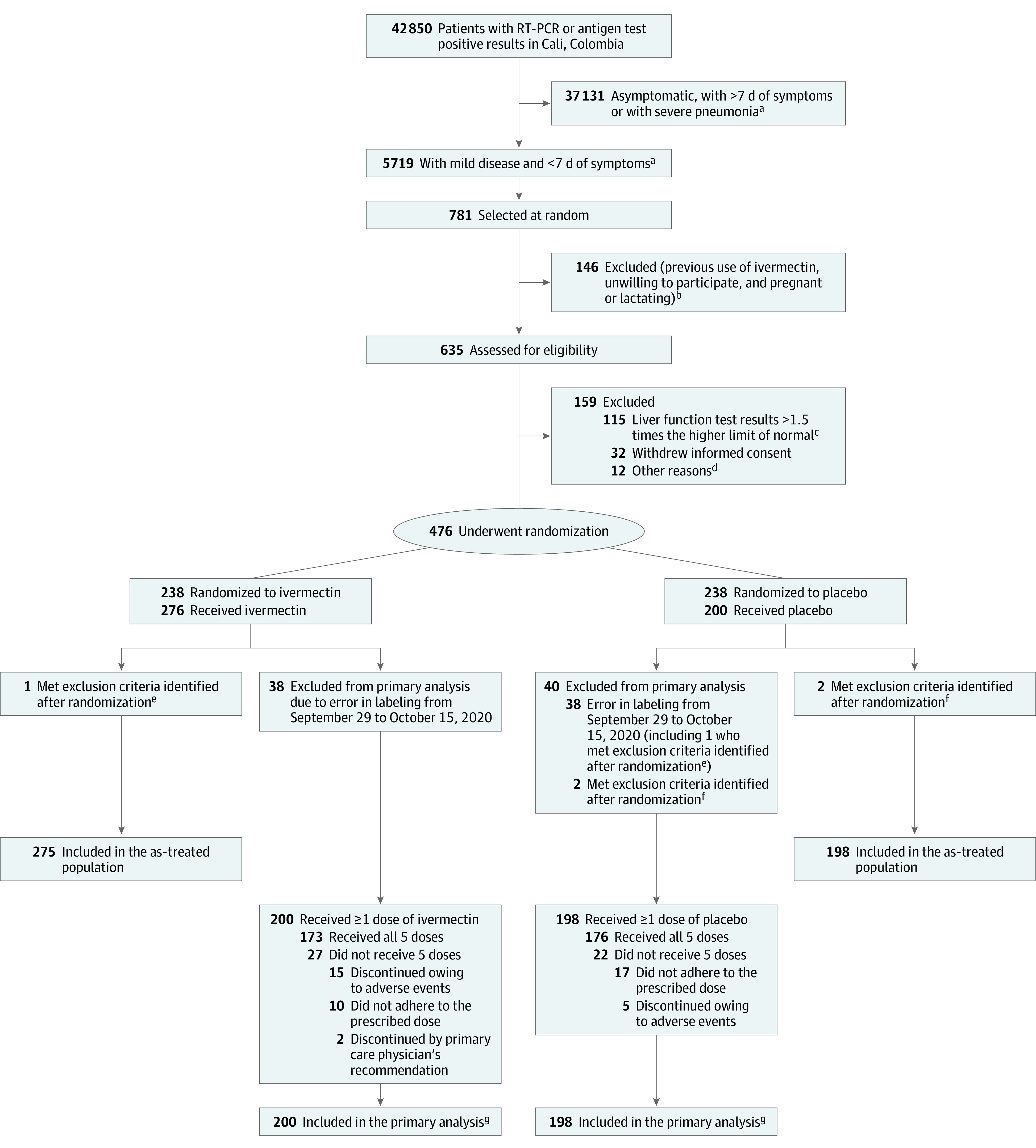
RT-PCR indicates reverse transcriptase–polymerase chain reaction.
aPatients with mild disease were at home or hospitalized but not receiving high-flow nasal oxygen or mechanical ventilation (invasive or noninvasive). Patients with severe pneumonia were receiving high-flow nasal oxygen, mechanical ventilation (invasive or noninvasive), or extracorporeal membrane oxygenation.
bThe numbers of patients with these exclusion criteria were not collected.
cAspartate aminotransferase and alanine aminotransferase.
dEight patients used ivermectin within 5 days prior to randomization, 1 had a positive pregnancy test, 1 was asymptomatic, 1 lived in an inaccessible area, and 1 had onset of symptoms 8 days prior to randomization.
ePatient was asymptomatic and was randomized to receive placebo but received ivermectin.
fUse of ivermectin before randomization.
gIncludes deaths and recoveries.
