Table 2.
Acid–base transitions in fluoroprolines.a
| ammonium moiety | carboxylic acid moiety | |||||
| cis-amide conformation | trans-amide conformation | |||||
| entry | structure | pKa | structure | pKa | structure | pKa |
| Pro | 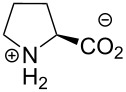 |
10.68 | 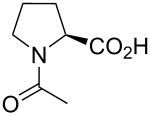 |
2.85 | 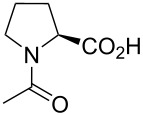 |
3.55 |
| R-Flp | 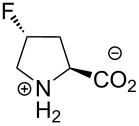 |
9.10 | 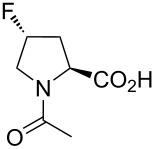 |
2.37 | 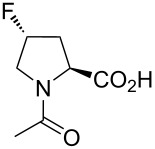 |
3.19 |
| S-Flp | 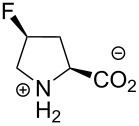 |
9.10 | 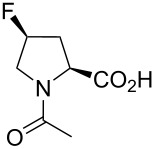 |
2.87 | 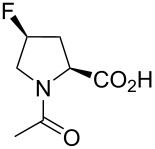 |
3.39 |
| Dfp | 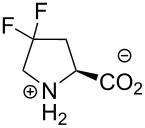 |
7.15 | 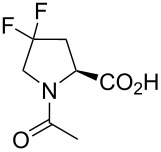 |
2.34 | 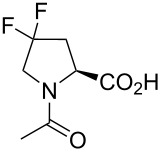 |
2.93 |
