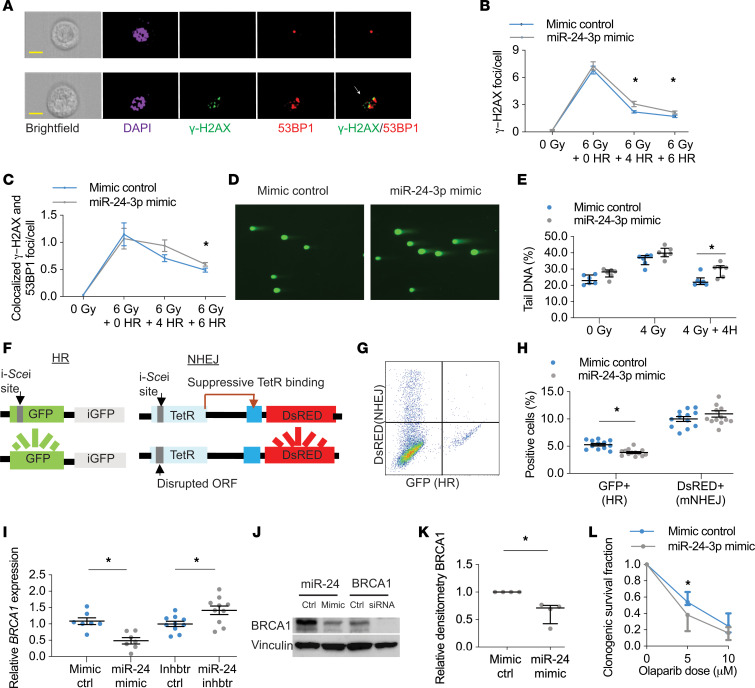
Figure 5. miR-24-3p inhibits HR and BRCA1.
(A) Imaging flow cytometry of cells exposed to 0 or 6 Gy of IR. Representative images of DAPI, γ-H2AX, and 53BP1 immunofluorescence staining. Colocalized γ-H2AX/53BP1 foci (yellow) are shown (arrow). Yellow scale bar: 10 μm. (B and C) Primary HAECs exposed to 0 Gy or 6 Gy with 0–6 hours (H) recovery (n = 7/group). (D and E) Comet assay in BEAS-2B cells exposed to 0 Gy, 4 Gy, or 4 Gy with 4H recovery. Percentage tail DNA reflects DNA damage (n = 6/group), with sample images following 4 Gy with 4H of recovery (original magnification, ×20). (F) Schema of DNA reporter cell assay with 2 integrated loci for measuring HR and mutagenic nonhomologous end joining (mNHEJ). (G) Representative flow cytometry demonstrating DsRED+ (mNHEJ) and GFP+ (HR) expression. (H) DNA reporter cells transfected with miR-24-3p mimic (n = 13/group) versus mimic control (n = 12/group). (I) BRCA1 expression (ΔΔCt of BRCA1/18S) measured by RT-PCR in BEAS-2B cells treated with miR-24-3p mimic versus mimic control (n = 7/group) and miR-24-3p inhibitor versus inhibitor control (n = 10/group). (J and K) Sample immunoblotting and relative densitometry of BRCA1/Vinculin in BEAS-2B cells treated with miR-24-3p mimic versus mimic control (n = 4/group). Sample immunoblotting includes siRNA against BRCA1 and siRNA control. (L) BEAS-2B cells transfected with miR-24-3p mimic versus mimic control and treated with olaparib at indicated dosages (n = 5/group). Error bars represent mean ± SEM (B, C, H, and I) or median ± IQR (E, K, and L). *P < 0.05 ordinary 1-way ANOVA (B, C, H, and I), Mann-Whitney (K), or Kruskal-Wallis (E and L) correcting for multiple comparisons using the 2-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. See complete unedited blots in the supplemental material.