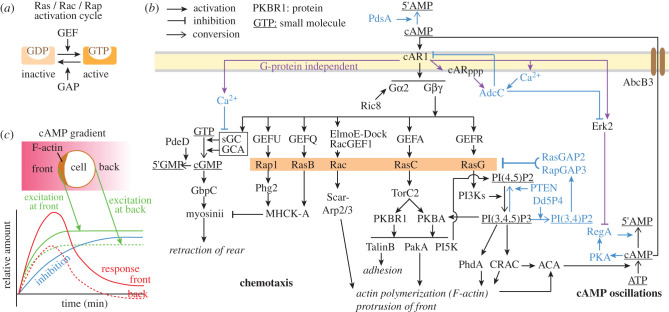
Figure 2.
Excitable networks in chemotaxis and morphogenetic signalling. (a) Activation cycle of small GTPase molecular switches. GTP: guanosine triphosphate; GDP: guanosine diphosphate; GAP: GTPase activating protein; GEF: guanine nucleotide exchange factor. (b) Chemotactic signal processing. See main text for explanation. Protein functions/abbreviations: AdcC: arrestin C; ACA: adenylate cyclase A; CRAC: cytosolic regulator of ACA; cAR1: cAMP receptor; cARppp: C-terminally phosphorylated cAR1; Gα2, β, γ: heterotrimeric G-protein subunits; Ric8: GEF for Gα2; PdsA and RegA: cAMP phosphodiesterases; PdeD: cGMP phosphodiesterase; sGC and GCA: guanylate cyclases; GbpC: cGMP binding protein; Phg2, TorC2, MHCK-A, PKBA, PKBR1, PakA, ERK2 and PKA: protein kinases; PI3K and PI5K: phosphatidyl-inositol kinases; PTEN and Dd5P4: phosphatidyl-inositol phosphatases; ElmoE-Dock: complex with GEF function. Scar: adaptor linking the Arp2/3 actin nucleation complex to Rac; PhdA: actin regulating PH-domain protein. cAMP/cGMP: 3′5′-cyclic adenosine/guanosine monophosphate; 5′AMP/5′GMP: 5′-adenosine/guanosine monophosphate; AbcB3: ABC transporter B3. Violet arrows: cAR1 activated responses that do not require a heterotrimeric G-protein. Blue text and arrows: components of negative feedback loops. (c) Local activation and global inhibition govern actin polymerization. The chemotactic response is best explained by a Turing type reaction–diffusion model. Here stimulation with attractant causes rapidly spreading excitation and more slowly spreading inhibition. At the front of the cell, excitation exceeds inhibition and actin is polymerized, while at the back inhibition is dominant. Redrawn in modified form from [6].