Fig. 7. Adult Fmr1 KO mice displays attention deficits with disrupted local/long-range input balance and hypernicotinic tone of ACAVIS neurons.
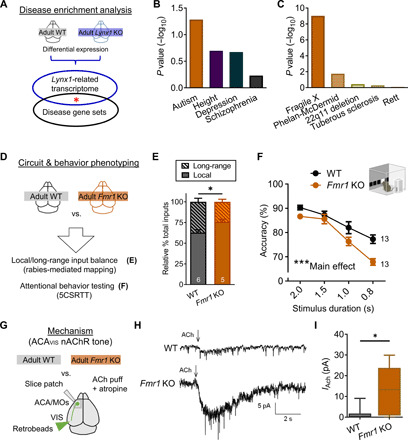
(A) Lynx1-related transcriptional signature (differential expression of adult Lynx1 KO and WT cortex) and GWAS gene sets were analyzed for enrichment. (B) Lynx1 KO signature mapped to GWAS gene sets (ASD: P = 0.0582; height: P = 0.2007; depression: P = 0.2127; schizophrenia: P = 1.0). (C) Lynx1 KO signature mapped to five autism-related gene sets [fragile X syndrome–related FMRP target genes: P = 9.7 × 10−10; Phelan-McDermid syndrome (Shank3KO): P = 0.0181; 22q11 deletion: P = 0.3664; tuberous sclerosis (Tsc2KO): P = 0.5036; Rett syndrome (Mecp2 null mutation): P = 0.8263]. (D) Adult Fmr1 KO mouse was screened for local/long-range input imbalance onto ACAVIS neurons and attentional deficits. (E) Rabies virus–mediated input mapping showed increased local input connectivity ratio for ACAVIS neurons in adult Fmr1 KO mice (t test, t9 = 2.715, *P = 0.0238; WT: five mice; Fmr1 KO: five mice). (F) Adult Fmr1 KO mice showed reduced accuracy with the 5CSRTT [two-way repeated measures ANOVA, main effect (genotype): F1,24 = 14.49, ***P = 0.0009; WT: 13 mice; Fmr1 KO: 13 mice]. (G) Adult Fmr1 KO mice were evaluated for their nicotinic tone of retrobeads labeled ACAVIS by whole-cell patch-clamp recordings. (H) Example trace recordings from ACAVIS neurons upon ACh puffing in the presence of atropine. (I) Adult Fmr1 KO mice show increased nicotinic responses in ACAVIS neurons [linear mixed model (rank-based), t6.94 = 3.40, *P = 0.0116; adult WT: 28 cells per 10 mice; Fmr1 KO: 19 cells per 7 mice).
