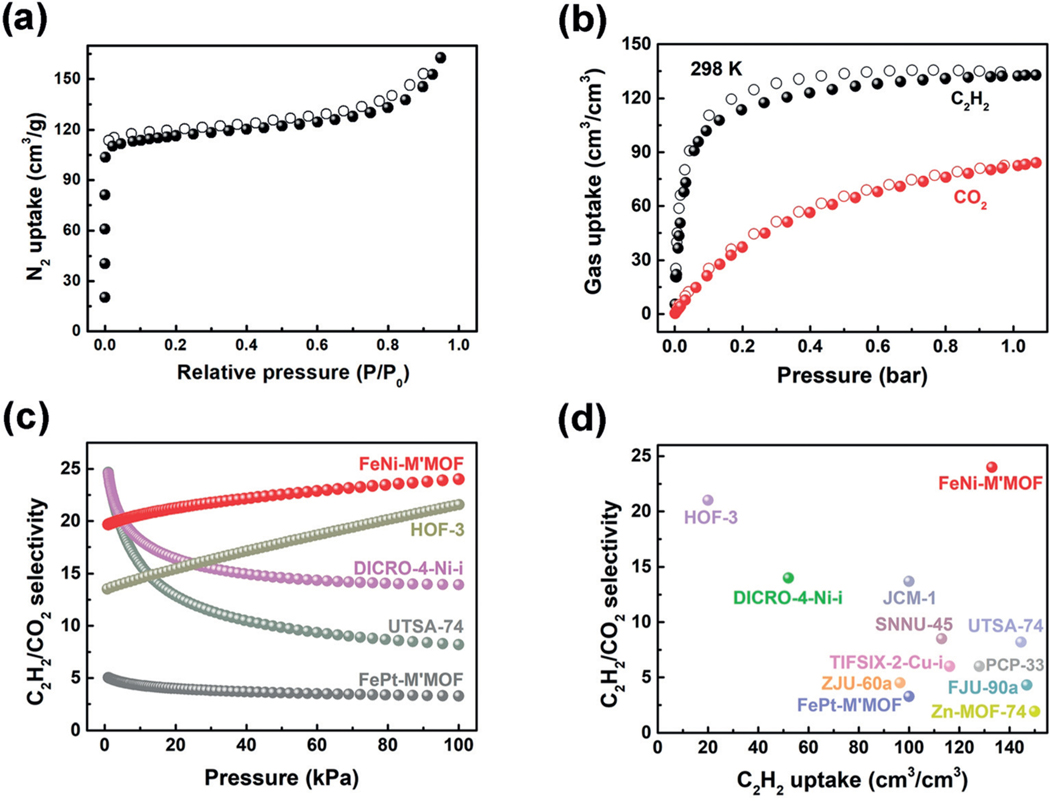
Figure 2.
a) N2 sorption isotherms for FeNi-M’MOF at 77 K. b) C2H2 and CO2 sorption isotherms for FeNi-M’MOF at 298 K. c) Comparison of IAST selectivities for equimolar C2H2/CO2 mixtures in FeNi-M’MOF, FePt-M’MOF and other materials in the range of 0–1 bar at 298 K. d) Comparison of C2H2/CO2 adsorption selectivity and volumetric C2H2 uptake at 1 bar in FeNi-M’MOF, FePt-M’MOF and other porous materials.