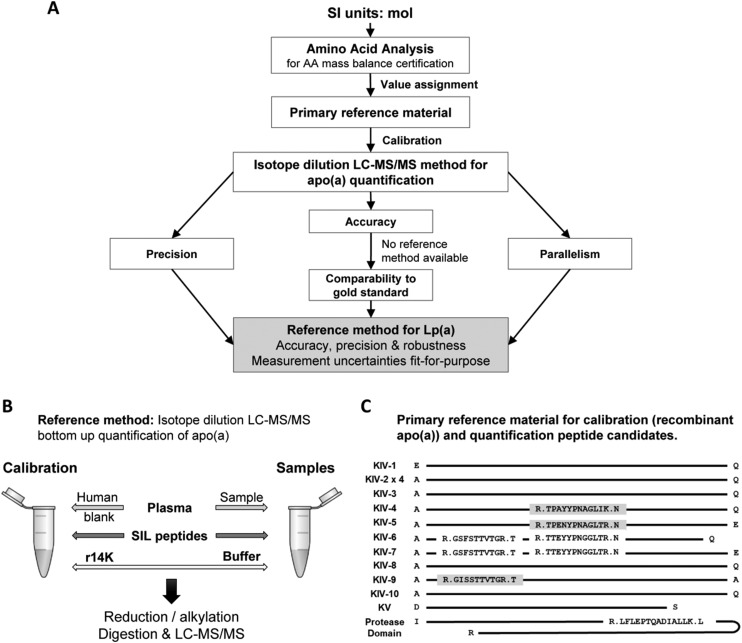
Fig. 1.
Development and validation of a reference method for the quantification of apo(a) in plasma. (A) Outline of the method development and validation. (B) Quantification strategy by targeted double isotope dilution LC-MS/MS outlining calibrators and sample preparation. (C) Sequence of the human recombinant apo(a) calibrator. Candidate quantification peptides and their locations are shown. Gray highlight, final quantification peptides; no highlight, candidate peptides not meeting quantification requirements.