Abstract
Indoleamine 2,3‐dioxygenase 1 (IDO1) is a key enzyme associated with immunomodulation through its regulation of the tryptophan‐kynurenine (Kyn) pathway in advanced cancers, including metastatic renal cell carcinoma (mRCC). However, the failure of IDO1 inhibitors when used in combination with immune checkpoint inhibitors (ICIs), as observed in clinical trials, raises a number of questions. This study aimed to investigate the association of tryptophan 2,3‐dioxygenase (TDO) and IDO1 with cancer development and resistance to immunotherapy in patients with RCC. In our analysis of RCC tissue samples, tissue Kyn levels were elevated in advanced‐stage RCC and correlated well with TDO expression levels in RCC tumor cells. In patients with mRCC, TDO rather than IDO1 was expressed in RCC tumor cells, showing a strong association with Kyn expression. Furthermore, immunohistochemical staining of TDO was strongly associated with the staining intensity of forkhead box P3, as well as ICI therapy response and survival in patients with mRCC. Our study is the first to show that TDO expression in tumor tissues is associated with progression and survival, confirming its potential as a predictive biomarker of primary resistance to immunotherapy in patients with mRCC. Our findings suggest that strategies aimed at inhibiting TDO, rather than IDO1, in combination with ICI therapy may aid in the control of mRCC progression.
Keywords: biomarker, IDO1, immune checkpoint inhibitor, renal cell carcinoma, TDO
This study aimed to investigate the association of tryptophan 2,3‐dioxygenase (TDO) and indoleamine 2,3‐dioxygenase 1 (IDO1) with cancer development and resistance to immunotherapy in renal cell carcinoma (RCC) patients. Our results suggest that TDO expression in tumor cells is associated with kynurenine accumulation, progression, and survival, confirming its potential as a predictive biomarker of primary resistance to immunotherapy in RCC patients. We believe that our study makes a significant contribution to the literature because, to the best of our knowledge, it is the first to show that the accumulation of kynurenine induced by TDO in tumor tissues is associated with progression and survival. Further, we believe that this paper will be of interest to the readership of your journal because our results strongly recommend the testing of specific TDO inhibitors or dual inhibitors of IDO1 and TDO with ICI treatment in future preclinical and clinical trials focusing on mRCC.
Abbreviations
- CI
confidence interval
- CPS
combined positive score
- FOXP3
forkhead box P3
- HPLC‐MS/MS
high‐performance liquid chromatography–tandem mass spectrometry
- ICI
immune checkpoint inhibitor
- IDO1
indoleamine 2,3‐dioxygenase 1
- KTR
Kyn/Trp ratio
- Kyn
kynurenine
- mAb
monoclonal antibody
- mRCC
metastatic RCC
- OS
overall survival
- PD‐L1
programmed cell death–ligand 1
- PFS
progression‐free survival
- RCC
renal cell carcinoma
- T/N
tumor/nontumor
- TDO
tryptophan 2,3‐dioxygenase
- Treg
regulatory T cell
- Trp
tryptophan
1. INTRODUCTION
Renal cell carcinoma (RCC) is the ninth most frequently reported cancer in Japan, accounting for approximately 3% of all cancers among adults and showing an annual increase in incidence. 1 One‐third of patients with RCC already have metastases at the time of cancer diagnosis. 2 Patients with metastatic RCC (mRCC) with a lack of response to vascular endothelial growth factor–targeted tyrosine kinase inhibitors or mammalian target of rapamycin inhibitors are more likely to have a poor prognosis in terms of tumor recurrence and metastasis. 3
Numerous recent studies have elucidated the key role of immune checkpoints in the modulation of immune responses in malignancies, including mRCC. 4 Therapy with immune checkpoint inhibitors (ICIs), including programmed cell death–ligand 1 (PD‐L1) and cytotoxic T‐lymphocyte–associated protein 4 blockers, has been demonstrated as the most promising approach for the activation of therapeutic antitumor immunity in various malignant diseases, including RCC. 5 However, the majority of patients are unlikely to benefit from the aforementioned therapies. Additionally, PD‐L1 expression has not been established as a clear‐cut exclusionary predictive biomarker, as it was found to be unrelated to improved response following ICI treatment in patients with mRCC. 6 , 7 Thus, there is a pressing need for novel potential targets for mRCC immunotherapy to be identified.
The kynurenine (Kyn) pathway is involved in the regulation of the immunosuppressive microenvironment in human tumors. 8 , 9 Tryptophan (Trp) degradation is thought to exert immunosuppressive effects partly through the direct effects of Trp catabolites and partly through Trp depletion, 10 and there is currently strong interest in the therapeutic targeting of key rate‐limiting enzymes, including indoleamine 2,3‐dioxygenase 1 (IDO1) and tryptophan 2,3‐dioxygenase (TDO protein encoded by the TDO2 gene). 11 Due to the role of Trp catabolism in promoting immune suppression, several small molecule inhibitors targeting Trp catabolism have been developed and are currently being tested in clinical trials. 11 , 12 Moreover, recent preclinical and clinical trials combining IDO1 inhibitors with ICIs have elicited high expectations of a positive impact in the field of immuno‐oncology through their synergistic effect on the restoration of antitumor immune responses in various tumors, including mRCC. 13 , 14 In contrast, the failure of the IDO1 inhibitor epacadostat when used in combination with pembrolizumab in a phase III clinical trial (ECHO‐301/Keynote‐252) on advanced melanoma and the negative clinical results pertaining to IDO inhibitor use observed in some studies 15 , 16 raise a number of questions. Data on the localization of IDO1 and TDO in RCC tumor tissues and their roles in immunosensitivity remain controversial. 17 , 18 Thus, it is important to systematically evaluate the association between protein expression and the localization of Kyn, as well as the relationship of TDO and IDO1 with tumor development and immunosensitivity in RCC.
In the present study, we aimed to clarify the significance of IDO1 and TDO expression involved in the Kyn pathway in RCC.
2. MATERIALS AND METHODS
2.1. Patient characteristics and study design
All experimental protocols were approved by the Institutional Review Board of the Fujita Health University School of Medicine (approval numbers: HM19‐265 and HM20‐209). Additionally, all methods were performed in accordance with the relevant local guidelines and regulations. An explanation was provided to the patients and a website with additional information and an opt‐out option was set up for the study.
Patients receiving treatment following a clinical diagnosis of RCC at the Fujita Health University Hospital between October 2016 and July 2020 were enrolled in this study. We assessed 66 consecutive patients for whom a tumor tissue sample and nontumor tissue sample, as well as pre‐ and/or postsurgical serum samples, had been preserved under frozen conditions.
In addition, the medical records of 40 patients with mRCC who received immunotherapy using ICIs within the fifth‐line setting were reviewed. All enrolled patients underwent cytoreductive nephrectomy (in the case of primary mRCC), nephron‐sparing surgery, or radical nephrectomy (for initially localized RCC); thus, primary RCC specimens were homogeneously available for immunohistochemical staining.
Safety assessments included physical examinations and laboratory tests that were performed the day before each nivolumab administration. Blood tests included those pertaining to hematology, renal and hepatic function, pancreatic enzymes, and hormones (thyroid function, adrenocorticotropic hormone, and cortisol). Data on treatment‐related adverse events, particularly immune‐related adverse events, as reported by each treating physician, were obtained from the patients’ clinical files and laboratory reports, and classified according to the Common Terminology Criteria for Adverse Events, version 4.0.
Disease assessments were performed by computed tomography or magnetic resonance imaging at baseline and then every 12 weeks as an institutional practice. A radiographic assessment was performed every 3 months and within 6 weeks of the original progressive disease (PD) to confirm tumor reduction, stability, or progression. Imaging data were evaluated by expert radiologists in a blinded manner according to the Response Evaluation Criteria in Solid Tumors, version 1.1, and categorized as: complete response (CR), partial response (PR), stable disease (SD), or PD. Objective response (OR) was defined as CR or PR. Treatment beyond progression was allowed in patients who derived an investigator‐assessed clinical benefit in the absence of rapid disease progression and were tolerant to the immunological treatment.
2.2. Tumor samples and regions
In 66 patients, tumor (n = 66) and nontumor regions (n = 66), as well as presurgical serum samples, were inventoried and immediately stored at −80°C after surgery until subsequent experiments. In addition, formalin‐fixed paraffin‐embedded RCC tissue blocks were obtained from the archives of the Department of Diagnostic Pathology, Fujita Health University Hospital, and were reviewed by uropathologists for diagnosis, Fuhrman grading, and Tumor‐Node‐Metastasis (2017) staging. A representative tumor block of each case was selected for further immunohistochemical analysis. Consecutive slides were used to allow the comparison of the same field of view in any given case.
2.3. Trp and Kyn measurements in tissue and serum samples
Trp and Kyn concentrations were determined by high‐performance liquid chromatography–tandem mass spectrometry (HPLC‐MS/MS) with an internal standard (2‐morpholinoethanesulfonic acid). All steps for tissue sample extraction were performed on ice. Tumor and nontumor samples were split into approximately 20‐mg quantities and transferred to clean microcentrifuge tubes, following which 10 μmol/L of 2‐morpholinoethanesulfonic acid in methanol (500 μL) was added. For tissue extraction, the samples were homogenized using an ultrasonic homogenizer (THU‐80; Az‐one), and then water (250 μL) and chloroform (400 μL) were added. After the mixture was centrifuged at 4°C and 15 000×g for 15 minutes, the supernatant was filtered using a 10‐kDa cutoff filter (Amicon Ultra centrifugal filter; Merck Millipore). The filtrate was lyophilized and stored at −80°C until HPLC‐MS/MS. The precipitate was dissolved in 50 μL of water and then transferred to a glass autosampler vial (P/N: 1030‐51,053; GL Science).
To 50 μL of serum, we added 10 μmol/L 2‐morpholinoethanesulfonic acid (internal standard) in methanol (500 μL), water (250 μL), and chloroform (400 μL). After mixing, the mixture was centrifuged at 4°C and 15 000×g for 15 minutes. Next, 200 μL of the resulting supernatant was filtered using a 10‐kDa cutoff filter (Amicon Ultra centrifugal filter). The filtrate was lyophilized and the precipitate was dissolved in 50 μL of water, after which it was subjected to HPLC‐MS/MS (LCMS‐8060; Shimadzu). Trp and Kyn were eluted from a reverse‐phase column (Discovery HS F5‐3 column [15 cm × 2.1 mm, 3 μm]) with the gradient method using a mobile phase with 0.1% formic acid in water and 0.1% formic acid in acetonitrile at a flow rate of 0.25 mL/min. We used the multiple reaction monitoring mode of MS/MS for Trp (m/z 205 > 188) and Kyn (m/z 209 > 192) detection.
For the analysis of the associations of Trp metabolism with the patients’ clinicopathological characteristics and survival, the Kyn/Trp ratio (KTR) in serum and tissue samples and the tumor/nontumor (T/N) ratio of Trp and Kyn were determined.
2.4. Immunohistochemistry and quantification of scoring for immune checkpoint molecules and regulatory T cells (Tregs)
Immunohistochemistry was performed as described previously. 19 Tissue samples were routinely fixed in 10% formalin and embedded in paraffin. Sections (3 µm thick) were cut and mounted on aminopropyltriethoxysilane‐coated slides. Sections were deparaffinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase was quenched with 0.03% hydrogen peroxide in methanol for 30 minutes at room temperature. Heat‐induced epitope retrieval was performed using a pressure pan (Delicio 6L; T‐FAL) for 10 minutes. Preliminary experiments determined the optimal soaking solutions for retrieving the antigenicity of the respective markers: 10 mmol/L citrate buffer (pH 6.0) for TDO and 1 mmol/L ethylenediaminetetraacetic acid (pH 9.0) for IDO1, PD‐L1, and forkhead box P3 (FOXP3). No pretreatment was required for Kyn. After pressure cooking, the sections were left to cool in the soaking solution for 30 minutes. Five primary antibodies were used to subtype the tumor and/or inflammatory infiltrate within the tumor microenvironment. The expression of immune checkpoint molecules was assessed using a rabbit monoclonal antibody (mAb) against PD‐L1 (Clone SP142, dilution 1:100; Abcam). IDO1 was detected using a rabbit mAb (Clone D5J4E, dilution 1:200; Cell Signaling Technology), TDO using a mouse mAb (Clone 4G2, dilution 1:100; OriGene Technologies, Inc), and Kyn using a mouse mAb (Clone 3D4‐F2, dilution 1:100; ImmuSmol SAS). Tregs were detected using an anti‐FOXP3 mouse mAb (Clone 236A/E7, dilution 1:100; Abcam). Histofine Simple Stain MAX PO (Nichirei Bioscience), employing the universal immunoperoxidase polymer method, was used as the secondary reagent. The reaction products were visualized by diaminobenzidine tetrahydrochloride (Agilent Technologies).
The degree of TDO expression was assessed and scored as: level 1, negative; level 2, containing some isolated TDO‐positive cells; level 3, containing diffuse regions enriched in TDO‐positive cells; and level 4, containing several regions with strong cytoplasmic staining of TDO‐positive cells. In addition, the degree of Kyn expression was assessed and scored in the same manner as that of TDO. The level of IDO1 expression was assessed in tumor cells, immune cells, and vessels, and was scored as: level 1, negative; level 2, 1%‐10%; level 3, 10%‐20%; and level 4, >20% positivity, as described by Seeber et al. 17 PD‐L1 expression was assessed using the combined positive score (CPS; including tumor cells and immune cells, regardless of staining intensity) as: CPS 1, <1%; CPS 2, ≥1% and <5%; CPS 3, ≥5% and <10%; and CPS 4, ≥10%. For FOXP3, systematic quantitative cell analysis was performed by manually counting the number of positive cells for each subset in up to five high‐power fields with hot spots of inflammation using the same field of view in consecutive slides. The total count of positive cells per high‐power field was calculated for each marker. All counts were performed by three independent observers with extensive experience in uropathology (MS, KaS, and YM) using a Zeiss Axio Imager 2 (40‐200× magnification). Each investigator repeated all counts twice, and the average value was used for statistical analysis.
2.5. Statistical analysis
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (The R Foundation for Statistical Computing). Descriptive statistics (absolute and relative frequencies for qualitative data; mean, standard deviation, and range for quantitative data) are given for all baseline and histopathological variables. Nonparametric Kruskal‐Wallis rank sum tests followed by Bonferroni‐corrected Mann‐Whitney U tests were used to compare mean ranks of different Kyn/Trp parameters according to pathological stage. Fisher's exact tests were used for between‐group comparisons. Correlations between parameters were assessed using Pearson's coefficient. Kaplan‐Meier analysis was performed to determine the effect on survival, and statistical differences were ranked according to the Mantel‐Cox log‐rank test. Multivariate Cox proportional hazards models were used to examine the variables associated with survival. A P‐value < .05 was considered significant.
3. RESULTS
3.1. Tissue Kyn T/N ratio rather than serum KTR is strongly associated with RCC stage
The baseline and histopathological characteristics of the 66 patients with RCC whose frozen serum and tissue samples were preserved are shown in Table 1. We first measured serum and tissue concentrations of Trp and Kyn. Kruskal‐Wallis rank sum tests showed that the tissue Kyn T/N ratio was strongly associated with RCC stage (P = .002; Figure 1A). Subanalyses using Bonferroni‐corrected Mann‐Whitney U tests revealed significant differences in the tissue Kyn T/N ratio between stage I and stage IV disease (P = .003). Conversely, the baseline serum KTR was not associated with RCC stage. An inverse correlation was observed between the serum KTR and RCC stage (Figure 1B); however, statistical significance was not reached (P = .627; Figure 1B). The tissue KTR (T/N ratio, P = .020; Figure 1C), but not the tumor KTR (P = .542; Figure 1D), was also associated with RCC stage, although the associations were not as strong as those for the tissue Kyn T/N ratio. One patient had pathological stage 0 disease after presurgical treatment with three courses of ipilimumab and nivolumab, and the Kyn T/N ratio decreased to 0.425.
TABLE 1.
Characteristics of 66 renal cell carcinoma (RCC) patients whose serum and tissue metabolites were analyzed
| Characteristic | Values |
|---|---|
| Age | |
| Mean (range) | 65.2 (45‐87) |
| Mean (IQR) | 67.0 (55.3‐72.8) |
| Gender n (%) | |
| Female | 16 (24.2) |
| Male | 50 (75.8) |
| Tumor side, n (%) | |
| Right | 27 (40.9) |
| Left | 39 (59.1) |
| Type of surgery, n (%) | |
| Radical nephrectomy | 22 (33.3) |
| Partial nephrectomy | 42 (63.6) |
| Biopsy | 2 (3.0) |
| Clinical T stage, n (%) | |
| T1a | 35 (53.0) |
| T1b | 20 (30.3) |
| T2a | 4 (6.1) |
| T3a | 5 (7.6) |
| T4 | 2 (3.0) |
| Clinical N stage, n (%) | |
| N0 | 63 (95.5) |
| N1 | 2 (3.0) |
| N2 | 1 (1.5) |
| Clinical M stage, n (%) | |
| M0 | 58 (87.9) |
| M1 | 8 (12.1) |
| Pathological stage (AJCC), n (%) | |
| 0 | 1 (1.5) |
| I | 50 (75.8) |
| II | 4 (6.1) |
| III | 3 (4.5) |
| IV | 8 (12.1) |
| Furman grading of RCC, n (%) | |
| Grade 1‐2 | 57 (86.4) |
| Grade 3‐4 | 6 (9.1) |
| NA | 3 (4.5) |
| Pathological type of RCC, n (%) | |
| Clear‐cell | 50 (75.8) |
| Papillary | 4 (6.1) |
| Chromophobe | 6 (9.1) |
| Others | 6 (9.1) |
Abbreviations: AJCC, American Joint Commission on Cancer; IQR, interquartile range.
FIGURE 1.
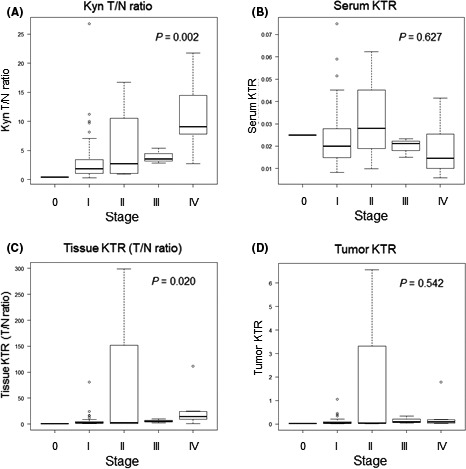
Tissue Kyn T/N ratio rather than serum KTR is strongly associated with RCC stage. Tumor regions and nontumor regions, as well as presurgical serum samples, from 66 patients with RCC were inventoried and Trp and Kyn concentrations were determined by HPLC‐MS/MS. The tissue Kyn T/N ratio (A), serum KTR (B), tissue KTR (T/N ratio; C), and tumor KTR (D) were calculated for each patient. Kruskal‐Wallis rank sum tests were performed according to pathological stage. HPLC‐MS/MS, high‐performance liquid chromatography–tandem mass spectrometry; KTR, Kyn/Trp ratio; Kyn, kynurenine; RCC, renal cell carcinoma; T/N, tumor/nontumor; Trp, tryptophan
3.2. TDO and Kyn are predominantly expressed in tumor cells while IDO1 is focally expressed in tumor endothelial cells
Next, we investigated the expression and localization of TDO and IDO1. While both are regarded as critical rate‐limiting enzymes in the metabolism of Trp to Kyn in various cancer cell types, their localization in tumor tissues and their functional significance remain controversial in RCC. 17 , 18 As shown in Figure 2A, both Kyn and TDO were expressed more strongly in RCC tissues than in adjacent normal renal tissues. The localization of TDO, but not IDO1, was shown to be very similar to that of Kyn, regardless of RCC stage. IDO1 was predominantly expressed in endothelial cells surrounding tumor cells (Figure 2A, 200× magnification). Representative stains for TDO scoring are shown in Figure 2B. Notably, TDO was strongly expressed in papillary RCC and collecting duct RCC, as well as clear cell RCC (Figure 2B).
FIGURE 2.
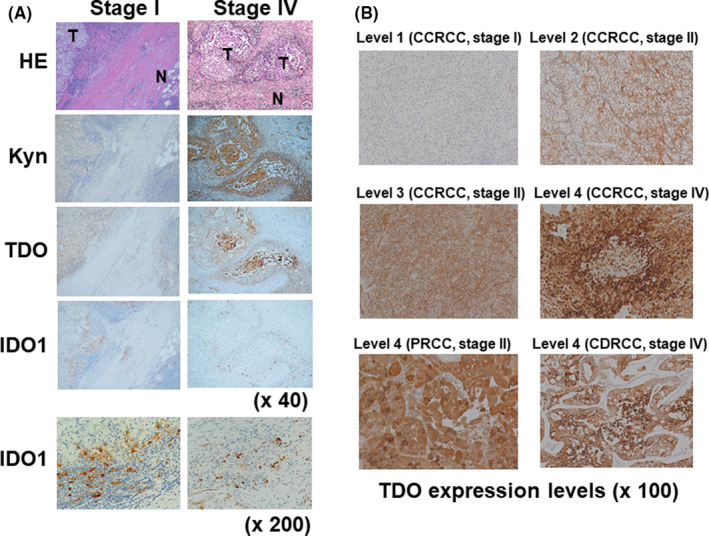
The expression of Kyn, TDO, and IDO1 in RCC tumor tissues. A, Representative images of stage I and stage IV RCC staining with specific antibodies for Kyn, TDO, and IDO1. B, Representative images of different TDO expression levels in CCRCC, PRCC, and CDRCC: level 1, negative for TDO; level 2, containing some isolated TDO‐positive cells; level 3, containing diffuse regions enriched in TDO‐positive cells; and level 4, containing several regions with strong cytoplasmic staining of TDO‐positive cells. CCRCC, clear cell RCC; CDRCC, collecting duct RCC; HE, hematoxylin‐eosin; IDO1, indoleamine 2,3‐dioxygenase 1; Kyn, kynurenine; PRCC, papillary RCC; RCC, renal cell carcinoma; TDO, tryptophan 2,3‐dioxygenase
3.3. Expression of TDO rather than IDO1 is associated with response to ICI treatment and correlates with infiltration of FOXP3‐positive cells, as well as Kyn expression
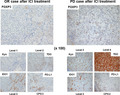
The immunohistochemical data suggest that TDO, but not IDO1, may be a key enzyme in the metabolism of Trp to Kyn in RCC tissues. Our results may provide important insights into the negative impact observed in recent clinical trials that used IDO1 inhibitors in combination with ICIs in patients with advanced RCC. 15 , 16 We, therefore, investigated the expression and localization of TDO, as well as IDO1, by immunohistochemistry in 40 patients with mRCC who received ICI treatment. We also evaluated the protein expression of Kyn using immunohistochemical analysis owing to the lack of availability of collective frozen mRCC tissue. We further investigated the expression of PD‐L1 and FOXP3 for the evaluation of the immunosuppressive environment in RCC tumor tissues. Representative images of the immunohistochemical analysis of patients with an OR and PD, respectively, after ICI treatment are shown in Figure 3. The baseline and histopathological characteristics of the primary RCC specimens, stratified by immunotherapy response, are shown in Table 2. Significant differences were observed between the three immunotherapy response groups in terms of Kyn, TDO, and FOXP3. Conversely, no significant differences with regard to IDO1 or PD‐L1 were observed. We further investigated the correlations between the expression levels of TDO or IDO1 and FOXP3 or Kyn in patients with mRCC. Pearson's coefficient analyses showed that TDO expression, but not IDO1 expression, was strongly correlated with Kyn expression (R = 0.561, P < .001; Figure 4A, B). Similarly, Mann‐Whitney U tests showed that the expression levels of FOXP3 were significantly increased in the TDO level 3‐4 group (P = .037), but not in the IDO1 level 3‐4 group (Figure 4C, D). Taken together, our results strongly suggest that the expression of TDO rather than IDO1 is correlated with Kyn expression in mRCC tumor cells. This may cause infiltration of FOXP3‐positive immune Tregs and resistance to ICI treatment.
FIGURE 3.
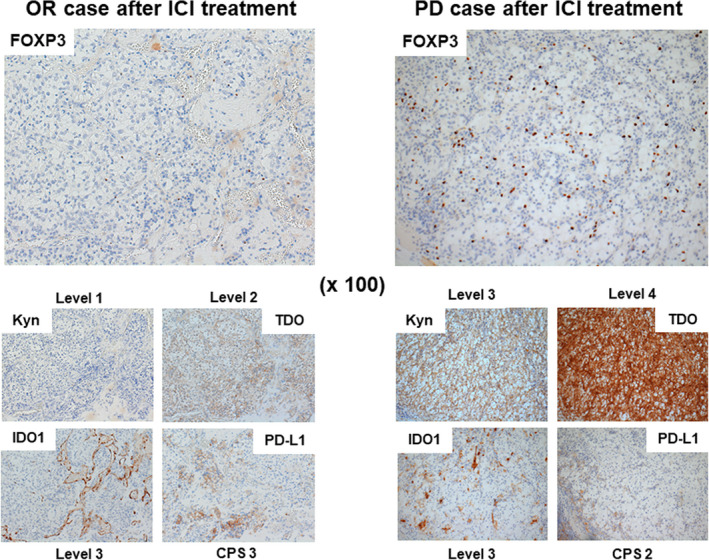
The expression of FOXP3, Kyn, TDO, IDO1, and PD‐L1 according to immunotherapy response in RCC tumor tissues. Representative images of the immunohistochemical analysis of patients with an OR and PD, respectively, after ICI treatment. PD‐L1 was expressed not only in tumor cells but also in immune cells. CPS, combined positive score; FOXP3, forkhead box P3; ICI, immune checkpoint inhibitor; IDO1, indoleamine 2,3‐dioxygenase 1; Kyn, kynurenine; OR, objective response; PD, progressive disease; PD‐L1, programmed death‐ligand 1; RCC, renal cell carcinoma; TDO, tryptophan 2,3‐dioxygenase
TABLE 2.
Characteristics of 40 mRCC patients according to ICI response groups
| Characteristic | OR (n = 12) | SD (n = 13) | PD (n = 15) | P value |
|---|---|---|---|---|
| Median age at ICI Treatment (IQR) | 68.0 (58.5‐72.8) | 73.0 (63.0‐79.0) | 65.0 (58.5‐75.0) | .660 |
| Gender, n (%) | ||||
| Female (n = 9) | 2 (16.7) | 3 (23.1) | 4 (26.7) | .895 |
| Male (n = 31) | 10 (83.3) | 10 (76.9) | 11 (73.3) | |
| ICI Treatment | ||||
| Nivolumab only (n = 32) | 8 (66.7) | 12 (92.3) | 12 (80.0) | .321 |
| Nivolumab and ipilimumab (n = 6) | 2 (16.7) | 1 (7.7) | 3 (20.0) | |
| Tyrosine kinase inhibitors and PD (L)‐1 Ab (n = 2) | 2 (16.7) | 0 (0.0) | 0 (0.0) | |
| Median ICI lines | 2.0 (1.0‐3.0) | 3.0 (2.0‐3.0) | 3.0 (2.0‐3.5) | .622 |
|
Median number of metastatic organs (IQR) |
2.0 (1.5‐3.0) | 2.0 (1.0‐2.0) | 2.0 (1.0‐2.0) | .399 |
| Pathological type | ||||
| CCRCC (n = 36) | 11 (91.7) | 11 (84.6) | 14 (93.3) | .821 |
| Others (n = 4) | 1 (8.3) | 2 (15.4) | 1 (6.7) | |
| Furman grading of RCC, n (%) | ||||
| Grade 1‐2 (n = 32) | 7 | 12 | 13 | .189 |
| Grade 3‐4 (n = 7) | 4 | 1 | 2 | |
| Not applicable (n = 1) | 1 | 0 | 0 | |
| Pathological T stage, n (%) | ||||
| pT1 (n = 13) | 3 (25.0) | 5 (38.5) | 5 (33.3) | .841 |
| pT2 (n = 8) | 2 (16.7) | 4 (30.8) | 2 (13.3) | |
| pT3 (n = 17) | 6 (50.0) | 4 (30.8) | 7 (46.7) | |
| pT4 (n = 2) | 1 (8.3) | 0 (0.0) | 1 (6.7) | |
| International Metastatic RCC Database Consortium risk classification | ||||
| Favorable (n = 8) | 3 (25.0) | 2 (15.4) | 3 (20.0) | .980 |
| Intermediate (n = 28) | 8 (66.7) | 10 (76.9) | 10 (66.7) | |
| Poor (n = 4) | 1 (8.3) | 1 (7.7) | 2 (13.3) | |
| Kyn levels, n (%) | ||||
| 1‐2 (n = 25) | 11 (91.7) | 8 (61.5) | 6 (40.0) | .026 |
| 3‐4 (n = 15) | 1 (8.3) | 5 (38.5) | 9 (60.0) | |
| TDO levels, n (%) | ||||
| 1‐2 (n = 25) | 10 (83.3) | 11 (84.6) | 4 (26.7) | .002 |
| 3‐4 (n = 15) | 2 (16.7) | 2 (15.4) | 11 (73.3) | |
| IDO1 levels, n (%) | ||||
| 1‐2 (n = 26) | 8 (66.7) | 7 (53.8) | 9 (60.0) | .919 |
| 3‐4 (n = 14) | 4 (33.3) | 6 (46.2) | 6 (40.0) | |
| PD‐L1 levels, n (%) | ||||
| CPS 1‐2 (n = 16) | 5 (41.7) | 5 (38.5) | 6 (40.0) | 1.000 |
| CPS 3‐4 (n = 24) | 7 (58.3) | 8 (61.5) | 9 (60.0) | |
| Median FOXP3 (IQR) | 3.5 (2‐8) | 5.0 (4.0‐6.0) | 7.0 (4.0‐8.0) | .042 |
Abbreviations: CCRCC, clear cell RCC; CPS, combined positive score; FOXP3, forkhead box P3; ICI, immune checkpoint inhibitor; IDO1, indoleamine 2,3‐dioxygenase 1; IQR, interquartile range; mRCC, metastatic RCC; OR, objective response; PD, progressive disease; PD‐L1, programmed cell death–ligand 1; RCC, renal cell carcinoma; stable disease (SD); TDO, tryptophan 2,3‐dioxygenase.
FIGURE 4.
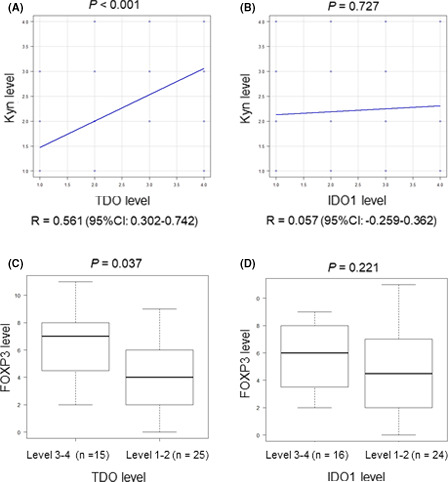
TDO rather than IDO1 is associated with the expression of Kyn and infiltration of FOXP3‐positive cells. Tumor tissues from 40 patients with mRCC were used to evaluate the correlation of expression of TDO, IDO1, Kyn, and FOXP3 by immunohistochemistry. Pearson's coefficient analysis showed that TDO expression levels (A), but not IDO1 expression levels (B), correlated well with Kyn expression levels. Mann‐Whitney U tests showed that FOXP3 expression levels were significantly increased in the TDO level 3‐4 group (C), but not in the IDO1 level 3‐4 group (D). CI, confidence interval; FOXP3, forkhead box P3; Kyn, kynurenine; IDO1, indoleamine 2,3‐dioxygenase 1; mRCC, metastatic RCC; TDO, tryptophan 2,3‐dioxygenase
3.4. TDO expression is strongly associated with ICI treatment–related survival in mRCC
Finally, we investigated whether the degree of immunohistochemical staining could aid in the evaluation of ICI treatment–related survival in patients with mRCC. In this study, the median progression‐free survival (PFS) was 7.2 (95% confidence interval [CI]: 5.1‐14.3) months, and the median overall survival (OS) after ICI therapy was 22.9 (95% CI: 16.3‐NA) months. The 1‐year OS rate (n = 27) was 80.4% (95% CI: 63.2‐90.2), which was similar to that obtained in a Japanese multi‐institutional study. 20 In the Kaplan‐Meier analysis of PFS according to ICI treatment, the TDO expression level 3‐4 group had significantly poorer prognoses (median PFS, 4.9 months; 95% CI: 1.4‐5.4) than the TDO expression level 1‐2 group (median PFS, 14.3 months; 95% CI: 7.0‐NA; P < .001; Figure 5A). In the corresponding analysis of OS, the TDO expression level 3‐4 group had significantly poorer prognoses (median OS, 14.8 months; 95% CI: 6.2‐NA) than the TDO expression level 1‐2 group (median OS, NA; 95% CI: 18.3‐NA; P = .040; Figure 5B). We performed multivariate analyses to identify predictors of PFS in patients who underwent ICI treatment for mRCC. In the univariate analysis, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≥1, TDO expression levels 3‐4, and FOXP3 counts were significantly associated with PFS. In the multivariate analysis, ECOG PS and TDO expression levels remained associated with PFS (Figure 5C).
FIGURE 5.
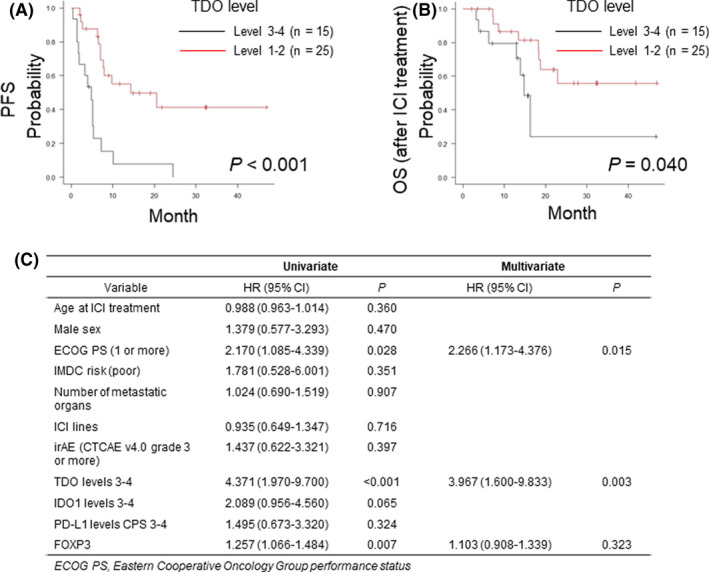
The association between TDO expression levels and ICI treatment–related survival in patients with mRCC. PFS (A) and OS (B) stratified by TDO expression levels (level 1‐2 vs. level 3‐4). P‐values were evaluated by a log‐rank test. C, Cox proportional hazards regression model for PFS. CPS, combined positive score; CTCAE, Common Terminology Criteria for Adverse Events; FOXP3, forkhead box P3; ICI, immune checkpoint inhibitor; IMDC, International Metastatic RCC Database Consortium; irAE, immune‐related adverse events; mRCC, metastatic renal cell carcinoma; OS, overall survival; PD‐L1, programmed cell death–ligand 1; PFS, progression‐free survival; TDO, tryptophan 2,3‐dioxygenase
4. DISCUSSION
In the present study, we demonstrated that the tissue Kyn T/N ratio was higher in patients with advanced‐stage RCC and was strongly correlated with TDO expression levels. We also observed that TDO expression was significantly associated with Treg infiltration and correlated with resistance to ICI treatment. To the best of our knowledge, the present study is the first to show that the TDO‐induced accumulation of Kyn in tumor tissues is associated with progression and survival, confirming its role as a possible predictive biomarker of primary resistance to immunotherapy in patients with mRCC. Surprisingly, our results that showed the predominant expression of TDO in RCC tumor cells is unexpected, since previous studies showed the expression of TDO in tumor tissues by real‐time polymerase chain reaction 9 or in macrophages, tumor endothelial cells, or pericytes in RCC tissues rather than tumor cells. 17 , 18
An important measure of Trp metabolism that has been indicated in the prognostication of different cancers is the serum KTR, which when elevated indicates that Trp is being metabolized through the Kyn pathway by IDO1 or TDO. Given that other researchers have reported a higher KTR in the serum of patients with advanced‐stage RCC, or in those with resistance to ICIs, 21 , 22 our finding pertaining to the strong maintenance of balanced levels of substrate to catabolite in plasma at baseline in patients with RCC is unexpected. It has been documented that increased TDO/IDO activity does not necessarily result in increased serum Kyn levels. 23 Li et al 22 showed that a higher KTR in serum during treatment is associated with poorer outcomes after nivolumab treatment. Our preliminary study showed that the serum KTR was not altered by ICI treatment in patients with mRCC (data not shown). A recent study reported results that were similar to ours in that there was no correlation between the serum KTR, cancer progression, and response to ICI treatment. 24 Further studies are needed to clarify the significance of serum KTR monitoring. Our results, which were obtained from HPLC‐MS/MS and immunohistochemistry of tumor tissues, have the potential to aid physicians in the decision‐making on ICI treatment in patients with mRCC.
IDO1 has been positioned as the main enzyme for immunomodulation by regulation of the Trp‐Kyn pathway in advanced human tumors. 25 , 26 A multicenter, open‐label phase I/II trial (ECHO‐202/Keynote‐037) focusing on RCC showed that the selective IDO1 inhibitor epacadostat in combination with pembrolizumab resulted in encouraging antitumor activity in advanced RCC, as well as melanoma. 14 Although that clinical study was based on the tumor‐promoting effects of IDO1, a controversial report by Seeber et al 17 showed a relationship between IDO1 expression and immunosensitivity in mRCC. They reported that the expression of IDO1 in tumor endothelial cells correlated with the therapeutic response to nivolumab followed by a better PFS, contrary to our results, which showed that IDO1 expression was not associated with ICI sensitivity in patients with mRCC. However, our results, which showed that tissue Kyn expression was closely associated with TDO expression, but not IDO1 expression, highlight the significance of TDO in the Trp‐Kyn pathway, resulting in tumor development and ICI resistance in patients with mRCC. These results, in addition to the poor results obtained in a previous phase III trial (ECHO‐301/Keynote‐252), raise fundamental questions regarding the benefits of using IDO1 inhibitors alone in the treatment of advanced cancers, including mRCC. One reason for the failure of this trial may be the fact that the tested IDO1 inhibitor does not cross‐inhibit TDO. Selective IDO1 enzyme inhibitors (eg, epacadostat and BMS‐986205) either compete with Trp for the catalytic site of IDO1 or bind to the enzyme with very high affinity. It has been reported that the 50% inhibitory concentrations for blocking TDO enzymatic activity by these inhibitors were >100‐fold higher than those for blocking IDO1 enzymatic activity. 27 , 28 The endogenous expression of TDO in various cancers may inhibit T cell activity in the tumor microenvironment and counteract the pharmacological effects of IDO1‐specific inhibitors. 29 Considering the difference in the localization between IDO1 and TDO in RCC tumor tissues, both IDO1 and TDO may play unique roles in cancer development and ICI treatment response in mRCC, although further efforts are needed to clarify this issue.
One of the major limitations of this study is its limited sample size and the retrospective evaluation of oncological results. The small sample size, resulting in statistical power limitations, must be considered, particularly in terms of the role of TDO in both ICI sensitivity and survival.
In conclusion, our results suggest that TDO may be a useful biomarker for the prediction of cancer development and ICI sensitivity in patients with RCC. TDO may support cancer metastasis and disease progression by influencing the degree of anticancer immunity or promoting growth. Our results should be considered in the planning of strategies involving Kyn pathway blockage in combination with ICI treatment for mRCC. Our results strongly recommend the testing of specific TDO inhibitors or dual IDO1 and TDO inhibitors with ICI treatment in future preclinical and clinical trials focusing on mRCC.
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We thank Ms Emi Bito at Fujita Cancer Center for her technical assistance with the sample and data collection.
Sumitomo M, Takahara K, Zennami K, et al. Tryptophan 2,3‐dioxygenase in tumor cells is associated with resistance to immunotherapy in renal cell carcinoma. Cancer Sci. 2021;112:1038–1047. 10.1111/cas.14797
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119‐1132. [DOI] [PubMed] [Google Scholar]
- 3. Tannir NM, Schwab G, Grunwald V. Cabozantinib: an Active Novel Multikinase Inhibitor in Renal Cell Carcinoma. Curr Oncol Rep. 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Couzin‐Frankel J. Cancer immunotherapy. Science. 2013;342:1432‐1433. [DOI] [PubMed] [Google Scholar]
- 5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD‐1/PD‐L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868‐876. [DOI] [PubMed] [Google Scholar]
- 7. Patel SP, Kurzrock R. PD‐L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847‐856. [DOI] [PubMed] [Google Scholar]
- 8. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour‐promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197‐203. [DOI] [PubMed] [Google Scholar]
- 9. Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3‐dioxygenase. Proc Natl Acad Sci U S A. 2012;109:2497‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379‐401. [DOI] [PubMed] [Google Scholar]
- 12. Cheong JE, Sun L. Targeting the IDO1/TDO2‐KYN‐AhR Pathway for Cancer Immunotherapy ‐ Challenges and Opportunities. Trends Pharmacol Sci. 2018;39:307‐325. [DOI] [PubMed] [Google Scholar]
- 13. Gomes B, Driessens G, Bartlett D, et al. Characterization of the Selective Indoleamine 2,3‐Dioxygenase‐1 (IDO1) Catalytic Inhibitor EOS200271/PF‐06840003 Supports IDO1 as a Critical Resistance Mechanism to PD‐(L)1 Blockade Therapy. Mol Cancer Ther. 2018;17:2530‐2542. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell TC, Hamid O, Smith DC, et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open‐Label Phase I/II Trial (ECHO‐202/KEYNOTE‐037). J Clin Oncol. 2018;36:3223‐3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jung KH, LoRusso P, Burris H, et al. Phase I Study of the Indoleamine 2,3‐Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC‐0919) Administered with PD‐L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin Cancer Res. 2019;25:3220‐3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO‐301/KEYNOTE‐252): a phase 3, randomised, double‐blind study. Lancet Oncol. 2019;20:1083‐1097. [DOI] [PubMed] [Google Scholar]
- 17. Seeber A, Klinglmair G, Fritz J, et al. High IDO‐1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci. 2018;109:1583‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann D, Dvorakova T, Stroobant V, et al. Tryptophan 2,3‐Dioxygenase Expression Identified in Human Hepatocellular Carcinoma Cells and in Intratumoral Pericytes of Most Cancers. Cancer Immunol Res. 2020;8:19‐31. [DOI] [PubMed] [Google Scholar]
- 19. Shiogama K, Onouchi T, Mizutani Y, Sakurai K, Inada K, Tsutsumi Y. Visualization of Neutrophil Extracellular Traps and Fibrin Meshwork in Human Fibrinopurulent Inflammatory Lesions: I. Light Microscopic Study. Acta Histochem Cytochem. 2016;49:109‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinata N, Yonese J, Masui S, et al. A multicenter retrospective study of nivolumab monotherapy in previously treated metastatic renal cell carcinoma patients: interim analysis of Japanese real‐world data. Int J Clin Oncol. 2020;25:1533‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucarelli G, Rutigliano M, Ferro M, et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol. 2017;35(461):461.e15‐461.e27. [DOI] [PubMed] [Google Scholar]
- 22. Li H, Bullock K, Gurjao C, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;10:4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011;41:1173‐1183. [DOI] [PubMed] [Google Scholar]
- 24. Botticelli A, Mezi S, Pomati G, et al. Tryptophan Catabolism as Immune Mechanism of Primary Resistance to Anti‐PD‐1. Front Immunol. 2020;11:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191‐1193. [DOI] [PubMed] [Google Scholar]
- 26. Godin‐Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3‐dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985‐6991. [DOI] [PubMed] [Google Scholar]
- 27. Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520‐3530. [DOI] [PubMed] [Google Scholar]
- 28. Labadie BW, Bao R, Luke JJ. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan‐Kynurenine‐Aryl Hydrocarbon Axis. Clin Cancer Res. 2019;25:1462‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terai M, Londin E, Rochani A, et al. Expression of Tryptophan 2,3‐Dioxygenase in Metastatic Uveal Melanoma. Cancers. 2020;12(2):405. [DOI] [PMC free article] [PubMed] [Google Scholar]


