FIGURE 1.
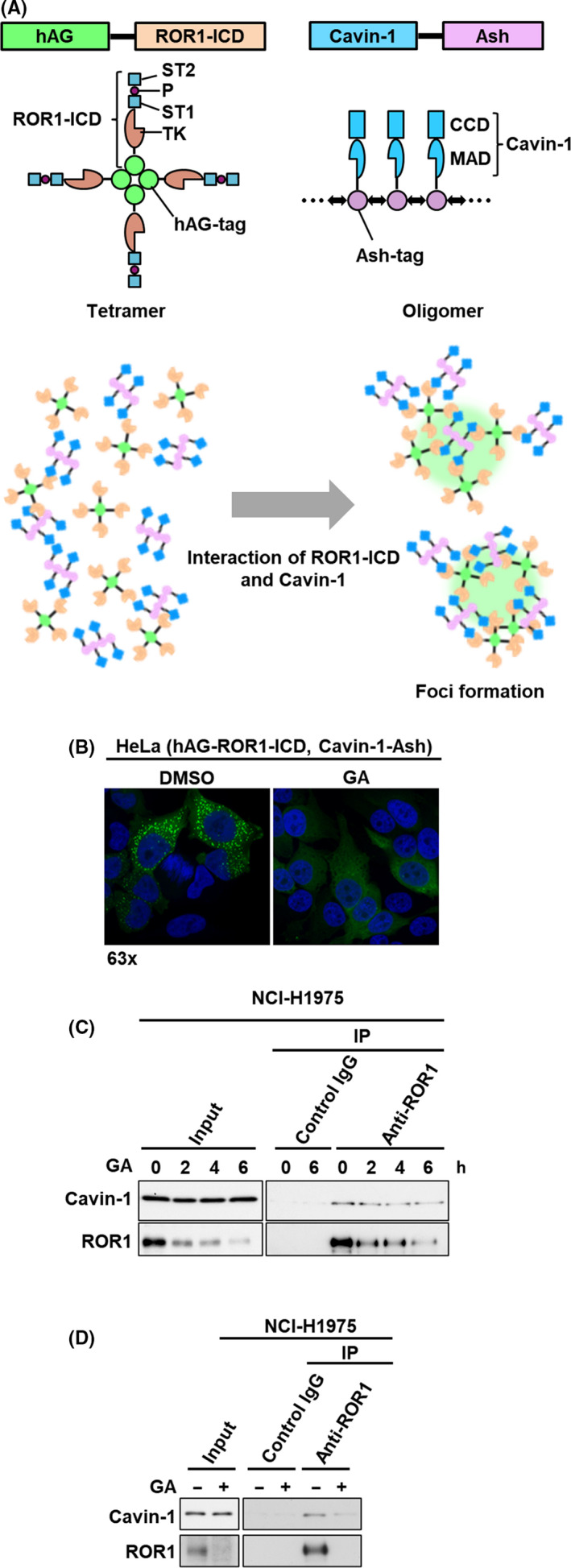
Identification of geldanamycin (GA) as a potential inhibitor of ROR1‐Cavin‐1 interaction. A, Schematic diagrams for constructs of humanized fluorescent Azami‐Green (hAG)‐ROR1‐intracellular domain (ICD), cavin‐1 assembly helper (Ash), and the fluorescent‐based technology detecting protein–protein interactions assay. ROR1‐ICD and cavin‐1 were genetically fused with tetramerizing hAG‐tag and oligomerizing Ash‐tag, respectively. A tetramer of hAG‐ROR1‐ICD and an oligomer of cavin‐1 can interact with multiple copies of each other, allowing the fluorescent proteins to form bright foci in cells. B, Fluorescent images of ROR1‐ICD and cavin‐1 interaction. Formation of fluorescent foci was suppressed after 24 h with 1 μmol/L GA (right panel) compared to control (left panel) in HeLa cells stably expressing hAG‐ROR1‐ICD and cavin‐1‐Ash. C, D, Immunoprecipitation‐western blot analyses using ROR1 Ab in NCI‐H1975 cells treated with 0.5 μmol/L GA for up to 6 h (C) and for 24 h (D). CCD, coiled‐coil domain; IP, immunoprecipitation; MAD, membrane association domain; P, proline‐rich domain; ST, serine/threonine‐rich domain; TK, tyrosine kinase domain
