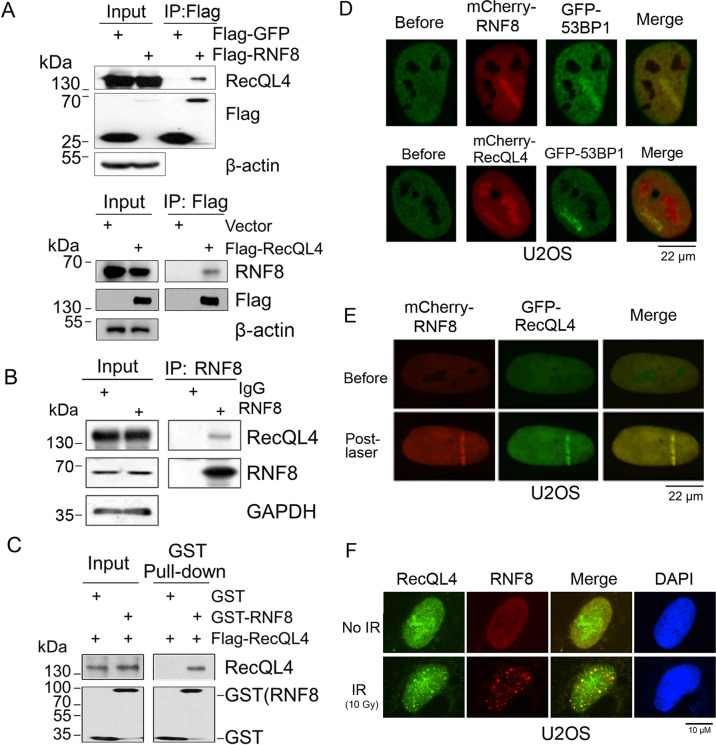
Fig. 1. RecQL4 physically interacts with RNF8.
A Endogenous RecQL4 or RNF8 were detected in anti-Flag immunoprecipitated fractions from Flag-RNF8/Flag-RecQL4 transfected U2OS cells by western blotting analysis. Flag-GFP/Empty vector-transfected cells were used as negative controls. B Interaction between endogenous RecQL4 and RNF8. Immunoprecipitated fraction from U2OS lysate was prepared using anti-RNF8 antibody (14112-1-AP, Proteintech), followed by western blotting analysis with anti-RecQL4 (25470002, SDIX) or RNF8 antibodies (sc-271462, Santa cruz). RecQL4 protein was detected in the RNF8 immunoprecipitated complex. C Direct interaction between RecQL4 and RNF8 was demonstrated by an in vitro pull-down assay. Purified recombinant GST-RNF8 was immobilized on Glutathione resin and incubated with purified Flag-RecQL4 in the IP buffer, and the bound proteins were examined by western blotting. D Colocalization of GFP-tagged 53BP1 and mCherry-tagged RNF8/RecQL4 at DSB track induced by UV micro-point laser. E Co-localization of mCherry-RNF8 and GFP-RecQL4 at DSB track induced by UV micro-point laser. F Co-localization of endogenous RNF8 and RecQL4 after X-ray irradiation. U2OS cells were exposed to 10 Gy of X-ray irradiation (25 mA, 160 kV; dose rate 0.995 Gy/min, X-RAD RS2000, Rad Source, USA), and fixed with 4% paraformaldehyde at 3.5 h post treatment. Indirect immunostaining was performed using primary anti-RecQL4 and RNF8 and fluorescence-dye conjugated secondary antibodies. After counterstaining with DAPI, images were captured using a fluorescence microscope (Leica DM5000 Microsystems).