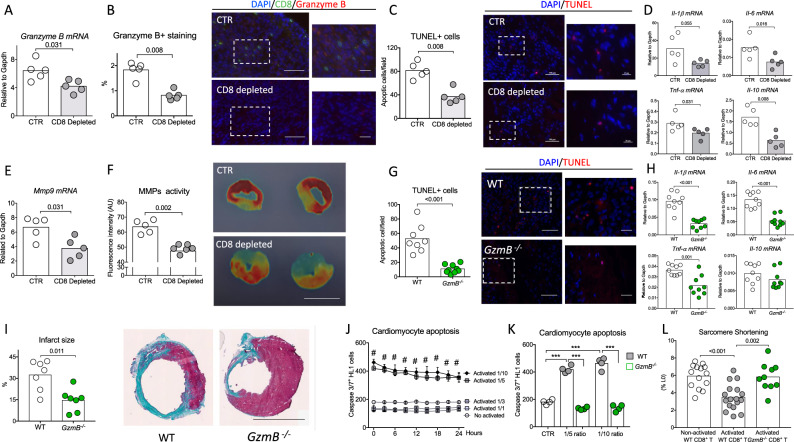
Fig. 4. CD8+ T lymphocyte depletion or Granzyme B global deficiency reduces cardiomyocyte apoptosis and pro-inflammatory responses within the ischemic heart tissue.
A Representative histograms of mRNA levels of Granzyme B within the injured myocardium on day 3 after MI in CTR (white) and CD8-depleted (gray) mice (n = 5/group). B Representative examples (right) and quantitative analysis (left) of Granzyme B staining in the ischemic heart of C57BL/6J mice with or without CD8 depletion (n = 5/group); scale bars 50 and 25 μm. C Representative examples (right) and quantitative analysis (left) of TUNEL+ cells (Red) in the peri-infarct area of C57BL/6J mice (n = 5/group); scale bars 50 and 25 μm. D Representative histograms of mRNA levels of Il-1β, I-6, Tnf-α, and Il-10 within the injured myocardium on day 7 after MI (n = 5/group). E Representative histograms of mRNA levels of Mmp9 within the injured myocardium on day 7 after MI (n = 5/group). F Quantification (left) and representative photomicrographs (right) of matrix metalloproteinase (MMP)-sense 680 activity in the ischemic heart measured by ex vivo reflectance epifluorescence imaging at day 7 (CTR n = 5 and CD8 depleted n = 6), scale bar 2.5 mm. G Acute MI was induced on C57bl6 wild-type (WT, white) mice or Granzyme B deficient (GzmB−/−, green) mice. Representative examples (right) and quantitative analysis (left) of TUNEL+ cells in the peri-infarct area of WT C57BL/6J or GzmB−/− mice at day 3 after MI (WT n = 8 and GzmB−/− n = 9); scale bars 50 and 25 μm. H Il-1β, Il-6, Tnf-α, and Il-10 mRNA levels measured by qPCR in infarcted heart at day 3 after MI (n = 9/group). I Representative photomicrographs (left) and quantitative analysis (right) of infarct size evaluation using Masson trichrome staining, in the 2 groups of mice (n = 7/group); scale bar 2.5 mm. J Purified non-activated or activated WT CD8+ T cells were co-cultured with cardiomyocytes at different ratios (Cardiomyocyte/CD8+ T cells) for 24 h before their removal. Apoptotic cardiomyocytes labeled with an active caspase-3 fluorescent dye was monitored for 24 h (n = 4–5/conditions), Data are presented as mean ± SEM, #P < 0.001 for activated CD8+ T cells 1/5 versus non-activated CD8+ T cells or activated CD8+ T cells 1/1 or activated CD8+ T cells 1/3; #P < 0.001 for activated CD8+ T cells 1/10 versus non-activated CD8+ T cells or activated CD8+ T cells 1/1 or activated CD8+ T cells 1/3. K WT or GzmB−/− CD8+ T cells were co-cultured with cardiomyocytes for 24 h at 1/5 and 1/10 ratio and cardiomyocyte apoptosis using an active caspase-3 fluorescent dye was quantified. Cardiomyocyte and non-activated CD8+ T cells co-culture was named CTR condition (n = 4/condition) ***P < 0.001. L Isolated cardiomyocytes were co-cultured overnight with CD8+ T cells isolated from WT or GzmB−/− mice and cardiomyocyte sarcomere shortening was measured (non-activated n = 14, activated n = 17 and GzmB−/− n = 10) at a ratio 1/3. P values were calculated using two-tailed Mann-Whitney test (A, B, C, D, E, F, G, H, I, J) or Kruskal-Wallis test (K, L). TUNEL, terminal deoxynucleotidyl transferase dUTP Nick End Labeling; MMP, matrix metalloprotease.