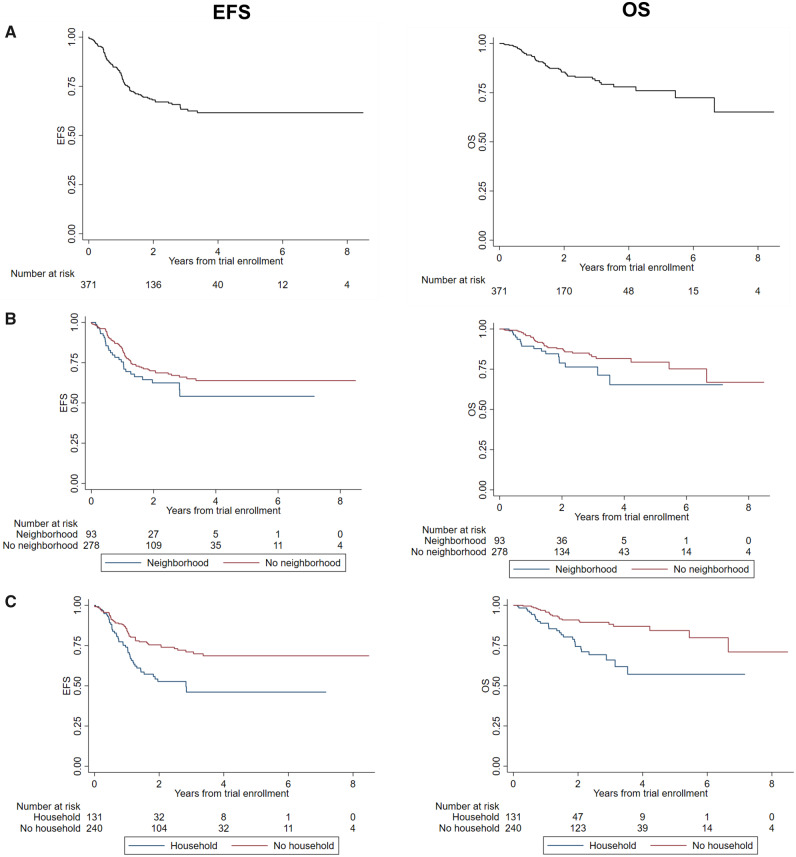
Figure 2.
Survival among children with high-risk neuroblastoma receiving targeted immunotherapy on Children’s Oncology Group (COG) protocols ANBL0032 or ANBL0931 at a Pediatric Health Information System (PHIS) center. Data are shown for Kaplan-Meier estimates of event-free survival (EFS) and overall survival (OS) for overall cohort from time of trial enrollment. Trial enrollment occurred after completion of both induction and consolidation therapy A). Two-year estimates (95% confidence interval [CI]): EFS = 67.9% (95% CI = 61.9% to 73.2%); OS = 85.5% (95% CI = 80.6% to 89.3%). B) Data for EFS and OS = stratified by neighborhood poverty group. Two-year estimates (95% CI): EFS no neighborhood poverty = 70.0% (95% CI = 63.1% to 75.8%) vs neighborhood poverty = 61.6% (95% CI = 48.5% to 72.3%), log rank test P = .15; OS no neighborhood poverty = 87.7% (95% CI = 82.2% to 91.6%) vs neighborhood poverty = 78.8% (95% CI = 66.6% to 87.0%), log rank test P = .07. C) EFS and OS stratified by household poverty group. Two-year estimates (95% CI): EFS no household poverty = 75.7% (95% CI = 68.8% to 81.2%) vs household poverty = 50.9% (95% CI = 39.4% to 61.3%), log rank test (P < .001); OS no household poverty = 90.9% (95% CI = 85.6% to 94.3%) vs household poverty = 74.4% (95% CI = 63.4% to 82.5), log rank test P < .001.