Abstract
Tibetan chickens are descendants of the ancestral red jungle fowl Gallus gallus. Very little is known about pathogens in Tibetan chickens living in the high-altitude environment. Here, we report for the first time the detection and isolation of avian leukosis virus from Tibetan chickens, with all the avian leukosis virus–positive samples belonging to subgroup J. Phylogenetic analysis of the sequence revealed these viruses were in a new branch compared with previous reports. The 3′-end of the pol gene in the new strains showed 8-amino acid deletion, with 2 strains displaying a large-scale deletion in the hr2 region of gp85 protein. Among all the strains, several mutations in the primer binding site leader sequence and untranslated region, which came from Rous-associated virus, were identified. It is interesting that some of these mutations may have contributed to the competitive advantages to these isolates as observed from their increased replication in vitro. These results indicated that the virus isolates from Tibetan chickens can have competitive advantage over the other strains circulating in the poultry population in future.
Key words: Tibetan chicken, ALV-J, sequence analysis, novel mutation
Introduction
Avian leukosis virus subgroup J (ALV-J) belongs to the family of retroviruses that can cause different tumors. It differs from the other 9 subgroups based on the identity of envelope protein, host range, and cross-neutralization patterns (Nair, 2020). Since its first isolation in the UK from the chicken with myeloma in 1988 (Payne et al., 1991), ALV-J has been reported all over the world. Avian leukosis virus subgroup J infection in chicken was found in the United States in 1997 (Fadly and Smith, 1999). Other countries such as France and Israel reported in 1992 (Hue et al., 2006) and 2002 (Davidson and Borenshtain, 2002), respectively. In 1999, four ALV-J strains were isolated from broiler chickens in Jiangsu, China (Du et al., 2000). Since 2004, there have been many reports on the identification of ALV-J in layer chickens (Xu et al., 2004; Gao et al., 2012). In 2009, a novel type of tumor, hemangioma, was observed in layer chickens, which was caused by a recombinant ALV-J strain (Wu et al., 2010). Nowadays, ALV-J is detected in almost all the regions and different indigenous breeds of chickens (Meng et al., 2018) throughout China. However, as with many other avian diseases, data are absent when it comes to Tibet. Owing to its unique geographical characteristics of high altitude, low pressure and oxygen concentration, and a less advanced transport system, the Tibetan chicken remains as the only breeder raised in Tibet (Zhang et al., 2007). Chickens from other regions have very limited chance to interact with native Tibetan chickens. Not only the appearance and living habits but also biochemistry and physiological features of Tibetan chickens are similar to the ancestors of domestic chickens, red gallus (Zhang et al., 2016). Tibetan chickens have many characteristics to allow them to survive in high-altitude conditions, such as cold resistance and responses to low pressure. Thus, this chicken is being introduced to other regions as breeders. As with other indigenous breeds of chicken, it will be interesting to explore the ALV status in Tibetan chickens. Here, we report our initial study on virus isolation and sequencing. The results may help to explain the evolutionary trend in Tibetan chickens and the basic characteristics of this virus.
In addition, ALV is mainly transmitted vertically from hens to embryos through the oviduct, and it could be a challenge to the human vaccine, which was developed from chicken embryos. To date, host range extension of ALV to mammalian cells due to mutation in env has been reported (Lounkova et al., 2017). Therefore, it is necessary to conduct research on ALV when the new virus strain was found.
Materials and methods
Clinical Samples and Cells
More than 2,000 eggs of Tibetan chickens were taken from Tibet to Taizhou, Jiangsu, China, where they were hatched and raised separately without interaction with other chickens. The hatchability of the chicken embryo was 85.2%, and the morbidity of 4-mo-old chickens was 1.5%. Some of the chickens showed a dramatic loss in weight and egg production. Their livers and spleens were enlarged several times. A total of 254 blood samples from these 13-wk-old chicken were sent to our laboratory for detection.
DF-1 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum and were kept in our own laboratory.
The ALV-J strain JS09GY3 was isolated from layer chickens (Wu et al., 2010) and was kept in our laboratory.
Virus Isolation
The virus isolation procedure was adapted from a previous study with further modification (Li et al., 2019). In brief, 60 μL of plasma from each whole-blood samples was inoculated into DF-1 cells in a 96-well plate. Two hours after inoculation at 37°C, the supernatant was replaced by Dulbecco's modified Eagle's medium containing 1% bovine serum. The DF-1 cells were then cultured at 37°C in a 5% CO2 atmosphere for 7 d. The cells were then tested using the p27 antigen detection kits developed in our laboratory (Zhou et al., 2019). The positive samples were named TBC-J with different numbers.
PCR and Sequencing
Avian leukosis virus–positive cells were collected, and proviral DNA was extracted as previously described by Bagust et al., 2004. Different subgroups can be identified through 2 pairs of primers, primers H5/H7 for ALV-J and primers H5/AD for ALV-A/B/E (Smith et al., 1998). The whole proviral genome sequence was amplified using 3 pairs of primers based on the sequence of the prototype ALV-J virus strain HPRS103. The primer sequences are shown in Table 1.
Table 1.
Primers used for sequencing and real-time quantitative PCR.
| Primer | Sequence | Length (bp) |
|---|---|---|
| H5 | GGATGAGGTGACTAAGAAAG | 545 |
| H7 | CGAACCAAAGGTAACACACG | |
| AD | GGGAGGTGGCTGACTGTGT | 326 |
| F1 | TGTAGTGTTATGCAATACTCTT | 2,554 |
| R1 | GCATGGGAATCCCCCTCCTA | |
| F2 | CGAATTCCCAGCGAAAATCT | 3,221 |
| R2 | CTTGATCATCCTTTTGGGTGATGT | |
| F3 | AGGTCGACCCCCGGTTAAGATACGAAT | 2,715 |
| R3 | TGAAGCCATCCGCTTCATGCAGGT | |
| gp37-F | TGCGTGCGTGGTTATTATTTC | 144 |
| gp37-R | AATGGTGAGGTCGCTGACTGT | |
| TBC-J6-F | GGTCAGGTGGTAATTGCACG | 178 |
| TBC-J6-R | GGCCCTCCCAAGGCATTAC | |
| 18s-F | TCAGATACCGTCGTAGTTCC | 145 |
| 18s-R | TTCCGTCAATTCCTTTAAGTT | |
| HMG14b-F | ACTGAAGAGACAAACCAAGAGC | 212 |
| HMG14b-R | CCAGCTGTTTTAGACCAAAGAATAC |
The PCR amplification condition for the whole genome included an initial denaturation cycle for 5 min at 95°C, followed by 30 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 50°C, and an extension for 3 min at 72°C, with a final extension for 7 min at 72°C. The PCR products were excised from 1.0% agarose gel, purified, and cloned into the TA vector pGEM-T easy (TaKaRa Co., Ltd., Dalian, China).
Three independent clones of each ALV-J isolate were sequenced by Beijing Genomics Institute (Beijing, China).
Phylogenetic Analysis
Whole-gene and partial gene sequences of each ALV-J strain were aligned for homology analysis of nucleic acids and amino acids, and the corresponding phylogenetic trees were constructed to evaluate their origin and evolution trend. These neighbor-joining trees were drawn using the MEGA X program (Temple University, PA;Arizona State University, AZ; Pennsylvania State University, PA), with confidence levels assessed using 1,000 bootstrap replications. The references with different background were chosen from GenBank.
Analysis of Replication Ability Through real-time quantitative PCR and 50% Tissue Culture Infective Dose
In brief, of 6-well cell culture plates with a monolayer of DF-1 cells, labeled A, B, and C, were prepared before infection. Plate A was inoculated with a dose of 10550% tissue culture infective dose(TCID50) of JS09GY3 each well, plate B was inoculated with a dose of 105TCID50 of TBC-J6 each well, and plate C was inoculated with a dose of 5 × 104TCID50 of JS09GY3 and 5 × 104TCID50 of TBC-J6 each well. At 1 to 6 d post infection (dpi), cell genomic DNA was extracted from one well of the infected cells from plate A and B, respectively, using the Genomic DNA Miniprep Kit (Axygen, California). Then, real-time quantitative PCR was performed to test the proviral load using the common primer pair for ALV-J env (gp37-F/R) and a primer pair exclusively for the env of TBC-J6 (TBC-J6-F/R). Chicken HMG14b, which has one copy in the chicken genome, was used as a reference (Su et al., 2018).
All culture supernatants of plate A and B were titrated for TCID50 using monoclonal antibody JE9, which is specific to gp85 of ALV-J (Qin et al., 2001). The results were analyzed using the Spearman–Kärber method. In addition, at 1 to 6 dpi, one well of the infected cells from plate C was harvested, and RNA was extracted using the Total RNA Miniprep Kit (Axygen, California). The RNA was reverse transcribed using the PrimerScript RT reagent Kit (Takara, Kusatsu, Japan). Then, quantitative reverse transcription PCR (qRT-PCR) was performed to test their competitive advantage, using the common primer pair for ALV-J env (gp37-F/R) and a primer pair exclusively for the env of TBC-J6 (TBC-J6-F/R). The results were normalized to the chicken housekeeping gene 18s RNA. The real-time quantitative PCR and qRT-PCR cycling conditions were as follows: 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 59°C for 34 s. Each experiment was performed independently for 3 times. Primers are shown in Table 1.
Results
Virus Detection and Isolation
The virus was isolated from DF-1 cells from Tibetan chickens. A total of 48 samples of the 245 tested samples were tested positive using ELISA kits. All positive samples produced a fragment of 545 bp in PCR with the ALV-J specific primer pair H5/H7, but not with primers H5/AD, which is for detection of ALV-A/B/E, indicating that all of them belonged to ALV-J. A total of 10 samples among them were randomly selected and sequenced for the whole proviral genome using the 3 pair of primers mentioned previously. The proviral genome of Tibetan chicken isolates ranged from 7,598 to 7,639 nucleotides in length.
Phylogenetic Analysis of ALV-J Isolates From Tibetan Chickens
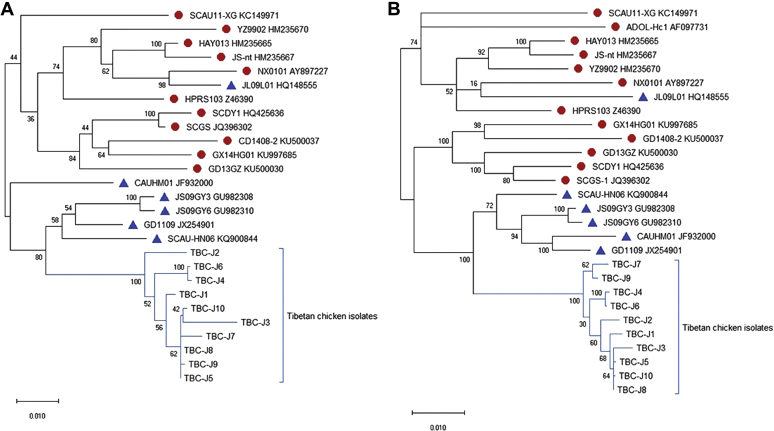
Phylogenetic analysis of ALV-J isolates from Tibetan chickens showed that these strains were clustered into a special branch. The isolates were 90.4 to 92.8% identical to the prototype strain of ALV-J HPRS103 and 90.4 to 93.3% identical to broiler chicken isolates in China. They were relatively closer to layer chicken isolates, and the homology ranged from 94.5 to 96.2% in provirus DNA, 95.6 to 97.4% in gag, 97.5 to 99.1% in pol, and 92.9 to 95.1% in env. Phylogenetic trees of the full-length and env gene are shown in Figure 1.
Figure 1.
Phylogenic analysis of new isolates of ALV-J. (A) Phylogenic analysis of the whole gene sequences. (B) Phylogenic analysis of env genes. Triangles represent layer chicken isolates, whereas circles represent broiler isolates, and others represent the new isolates. Abbreviation: ALV-J, avian leukosis virus subgroup J.
Characteristics of Coding Regions of ALV-J Isolates From Tibetan Chickens
Full-length pol protein consisted of 873 amino acids. It is worth notable that 90% (9/10) of ALV-J isolates from this study had a mutation from G to A at the residue 865, which resulted in a stop codon and 8-amino acid truncation in advance.
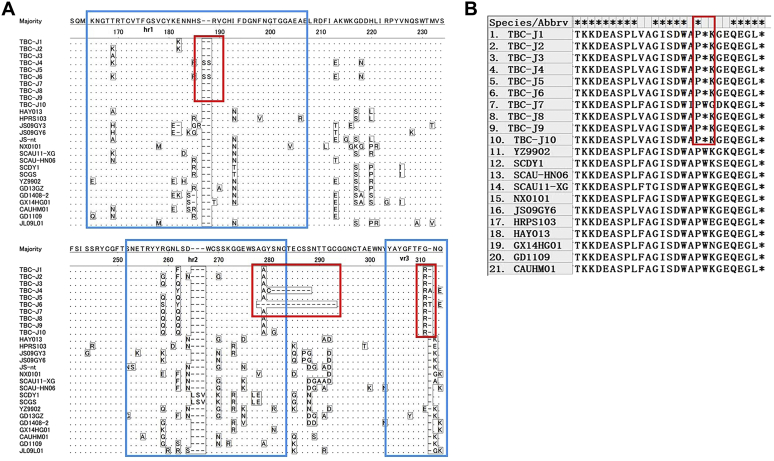
The env gene of Tibetan chicken isolates ranged from 1,464 to 1,512 nucleotides long, and nucleotide changes occurred throughout the env gene, mainly in hr1, hr2, and Vr3, including insertion, deletion, and replacement mutation. Importantly, 2 strains from this study, TBC-J4 and TBC-J6, had a large scale of deletion in hr2 of gp85 position. The former showed 8-amino acid deletion from residue 221 to 228, and the latter displayed 16-amino acid deletion from residue 219 to 234. Those 2 strains also had a double serine insertion at the position 186 and an alanine or threonine insertion at the position 310. Mutations in pol and envgenes are shown in Figure 2.
Figure 2.
Mutations in the coding sequences of ALV-J. Blue squares represent hr1, hr2, and vr3 of ALV-J, and red squares represent main mutations. The dots indicate identical residues, the letters indicate substitutions, and the dashes indicate deletion (A) mutation in gp85 of ALV-J isolates and (B) mutation in pol of ALV-J isolates. Abbreviation: ALV-J, avian leukosis virus subgroup J.
The gag gene encodes structural and nonstructural proteins. Among all the proteins, p2 was the most conserved, followed by p15 and p27, whereas p19, p10, and p12 of Tibetan chicken isolates had several mutations, such as I113 A T144 A and Q213P.
Characteristics of Noncoding Genes of ALV-J Isolates From Tibetan Chickens
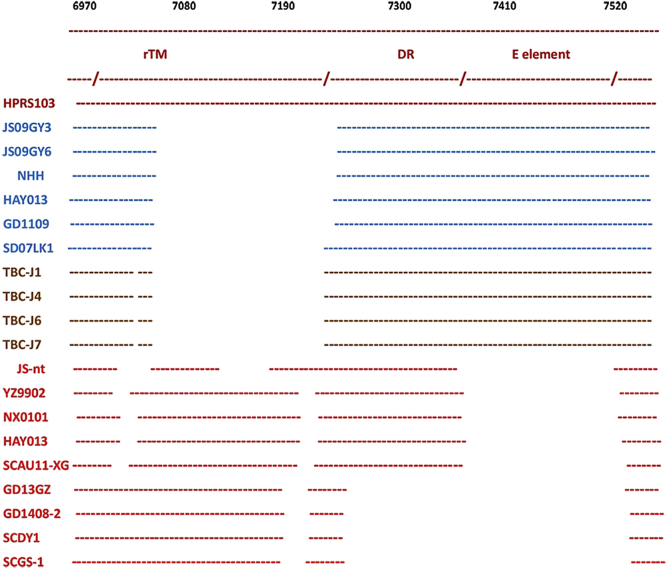
The primer binding site (PBS) and untranslated region (UTR) of all strains from both this study and GenBank were analyzed. The UTR of ALV-J isolates from this study had a deletion of 205 bp in r-TM and DR-1 (175 bp in r-TM, 30 bp in DR-1). Interestingly, this is the same deletion reported in the layer chicken isolates nationwide. The insertion of a 20-bp sequence (from 7,351–7,550) in the N terminal of r-TM and a 20-bp sequence in the C terminal of DR-1 (from 7,421–7,440) was identified from RAV-1. Homology of the PBS among different strains was high, ranging from 95.1 to 100% in broiler chicken isolates and 90.1 to 93.2% in layer chicken isolates in comparison with HPRS103. The PBS of Tibetan chicken isolates showed higher homology with layer chicken isolates, from 94.2 to 95.7%. A 19-bp insertion was found between the position 574 and 575 (Figure 3).
Figure 3.
Comparison of 3′UTR of ALV-J isolates. Blue represents layer chicken isolates, whereas red represents broiler chicken isolates, and others represent the new isolates. Blue squares represent sequences from Rous-associated virus. Abbreviations: ALV-J, avian leukosis virus subgroup J; UTR, untranslated region.
Viral Replication and Competitive Advantages of the Isolates From Tibetan Chickens
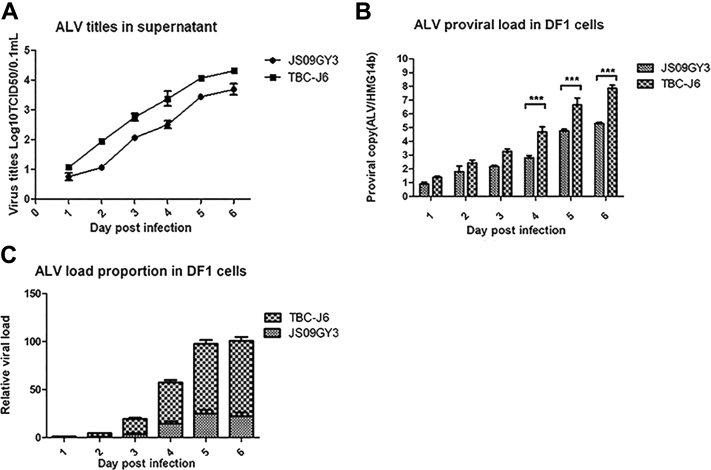
Supernatants of the infected cells at different intervals were harvested and tested for viral titers using the Spearman–Kärber method. TBC-J6 showed a higher virus titer than JS09GY3 from 1 to 6 dpi, as shown in Figure 4A.
Figure 4.
Comparison of proliferation and competitive advantages of different strains. (A) Supernatants of different infected cells were harvested at 1 to 6 d after infection and then titered for 50% tissue culture infective dose (TCID50) using the Spearman–Kärber method. (B) DNA of the infected cells was extracted at 1 to 6 d after infection. The proviral genome load in DF-1 cells was detected by real-time quantitative PCR. HMG14b is used as a reference. (C) RNA of the cells coinfected with 2 viruses was extracted at 1 to 6 d after infection. Quantitative reverse transcription PCR was performed to test the expression of the 2 viruses at different intervals, with 18s RNA used as a reference.
The load of the proviral genome in the chicken genome was tested using the single-copy housekeeping gene, HMG14b, as a reference. It is shown that TBC-J6 had more copy number in the cell genome than JS09GY3 at different times (Figure 4B). At 5 and 6 dpi, each cell could have more than 7.83 copies of TBC-J6 on average, but only 5.27 copies of JS09GY3 in genomic DNA.
Finally, the competitive advantage of the Tibetan isolates was compared through coculturing cells with different strains at the same time. The replication of different strains was detected every day by qRT-PCR (Figure 4C). The results showed that from 1 to 6 dpi, mRNA levels of both TBC-J6 and JS09GY3 were increased, but the rate of increase of TBC-J6 was much faster (121 times vs. 62 times, respectively). TBC-J6 always occupied about 65 to 85% of total transcripts in coinfection.
Discussion
Tibet has a distinct ecosystem and microbiota with unique characteristics because of geographical isolation. To use animal resources in Tibet, we should also pay attention to the potential indigenous pathogens. Those pathogens could pose a great threat to health and economic development. No report was available concerning the epidemiology of ALV in Tibet until now. This study first reported the detection and gene features of ALV in Tibetan chickens. It can help us to know about the status of ALV in the Tibetan poultry population and provide clues about the evolution trend of this virus.
With phylogenetic analysis of both the whole genome and distinct set of genes, we found that all 10 ALV strains isolated from Tibetan chickens belonged to ALV-J and clustered into a single new branch, in comparison with those from broiler chicken isolates and layer chicken isolates. In the perspective of molecular characteristics, ALV-J from Tibetan chickens exhibited unique features in its whole proviral genome, especially in pol, env, and UTR.
Gag and pol of ALV are usually conserved in almost all the ALV strains; however, we found novel mutations in pol genes from Tibetan chicken isolates. In the C-terminal of the pol gene, a mutation from TGG to TGA gave rise a stop codon that resulted in an 8-animo acid truncation in the pol fusion protein. Indeed, the pol precursor usually undergoes protease cleavage during maturation, and the 35 amino acids in the 3′ end will be removed. It needs to be investigated whether there are some additional functions for this region in vivo, although it appears to be not essential for virus replication, at least in vitro (Katz and Skalka, 1988).
The env genes have important roles in the distinct pathogenicity, tumorigenicity, and host range of these viruses (Munguia and Federspiel, 2019). Sequence analysis suggested that Tibetan isolates were highly homologous to layer chicken isolates, compared with HPRS103 and Chinese broiler chicken isolates. However, Tibetan chicken isolates still show many different features, indicating their distinct characteristics. Previous reports have identified the hr1 and hr2 domains are responsible for the ligand–receptor interaction directly (Swanstrom et al., 2017; Federspiel, 2019), while vr3 can recognize the specific receptor (Holmen et al., 2001). Some studies have demonstrated that a single mutation (L154S) in gp85 of ALV-B is sufficient for the extended host range to nonavian cell types (Melder et al., 2003). This indicated that gp85 is essential for the fitness of ALV to the environment, and even some small changes can influence the virus characteristics. Except NHE1, there were reports identifying ANXA2 (Mei et al., 2015) and GRP78 (Wang et al., 2016) as the receptors for ALV-J when studying on layer chicken isolates. Although it had been identified that residues 28 to 39 of chNHE1 were the key position mediating the ALV-J invading cells (Guan et al., 2018), we do not know the minimal amino acid residues of gp85 that binds to the receptor. Here, we found a large-scale deletion about 8 or 16 amino acids in gp85 of ALV-J. Because the large-scale deletion in gp85 did occur in ALV-J from field cases and the retroviral host range extension with env-activating mutations resulting in receptor-independent entry was also found (Lounková et al., 2014), we should investigate the function of hr2 in future.
Analysis of the noncoding region is essential for studying the evolution of ALV-J. The evidence obtained here indicated that Tibetan chicken isolates were possible recombinants between ALV and RAV-1, both in the PBS and UTR. The 19-bp insertion in the PBS was from RAV-1 and only found in several strains (Lai et al., 2011; Pan et al., 2012). Recently, the19-bp insertion was proved to promote the replication of ALV (Ji et al., 2014). The UTR is the region prone to mutate owing to the instability of reverse transcription and gene recombination (Nichol, 1996). Tibetan chicken isolates have the deletion in the UTR like most layer isolates, except for the 2 different sequences in r-TM and DR-1. The UTR contains many potent regulatory sequences that are essential in viral replication. r-TM was considered to be not a necessity for virus replication, whereas DR-1 could help the virus to replicate as a transport element (Zavala et al., 2007). E element is related to the host type and viral replication (Chesters et al., 2006) and is believed to be involved in encoding virus-encoded microRNAs recently (Yao et al., 2014). However, the specific function of the two 20-bp sequences inserted in r-TM and DR-1 still needs further investigation.
It is interesting that these mutant strains such as TBC-J6 can proliferate much faster than JS09GY3 in vitro as indicated by the levels of the viral titer in the supernatants. In addition, when we compared the copy numbers of the proviral genomes of the two viruses, it was found that TBC-J6 can integrate more copies in the chicken genome. We also tested whether there is any competitive advantage for TBC-J6 in virus coculture systems. The results showed that TBC-J6 had overwhelming advantages all the time. These results suggested that these mutations of isolates can greatly enhance the replication ability of the virus.
It is not clear how these viruses may have originated in Tibetan poultry. The overall sequence similarity to the nationwide ALV-J isolates suggested that these have been introduced from other parts of China. However, distinct phylogenetic grouping of these isolates suggested that they evolved to establish as a niche in the distinct Tibetan environment. Although further studies are required to explore this, it is possible that the competitive advantage demonstrated in this study has contributed to these isolates becoming the dominant population in the Tibetan population.
Acknowledgments
These studies were supported by grants from NCFC-RCUK-BBSRC (grant no. 31761133002 and BB/R012865/1), China and United kingdom; the Ministry of Science and Technology (grant no. 2016YFD0500803, 2017YFD0500702), China, and Jiangsu natural science research project (19KJD230001), China.
Disclosures
All the authors have no competing interests with regard to the publication of the data in this manuscript.
References
- Bagust T.J., Fenton S.P., Reddy M.R. Detection of subgroup J avian leukosis virus infection in Australian meat-type chickens. Aust. Vet. J. 2004;82:701–706. doi: 10.1111/j.1751-0813.2004.tb12163.x. [DOI] [PubMed] [Google Scholar]
- Chesters P.M., Smith L.P., Nair V. E (XSR) element contributes to the oncogenicity of Avian leukosis virus (subgroup J) J. Gen. Virol. 2006;87:2685–2692. doi: 10.1099/vir.0.81884-0. [DOI] [PubMed] [Google Scholar]
- Davidson I., Borenshtain R. The feather tips of commercial chickens are a favorable source of DNA for the amplification of Marek's disease virus and avian leukosis virus, subgroup J. Avian Pathol. 2002;31:237–240. doi: 10.1080/03079450220136549. [DOI] [PubMed] [Google Scholar]
- Du Y., Cui Z., Qin A., Silva R.F., Lee L.F. Isolation of subgroup J avian leukosis viruses and their partial sequence comparison. Chin. J. Virol. 2000;16:341–346. [Google Scholar]
- Fadly A.M., Smith E.J. Isolation and some characteristics of a subgroup J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States. Avian Dis. 1999;43:391–400. [PubMed] [Google Scholar]
- Federspiel M.J. Reverse Engineering provides Insights on the evolution of subgroups A to E avian sarcoma and leukosis virus receptor Specificity. Viruses. 2019;11:497. doi: 10.3390/v11060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yun B., Qin L., Pan W., Qu Y., Liu Z., Wang Y., Qi X., Gao H., Wang X. Molecular epidemiology of avian leukosis virus subgroup J in layer flocks in China. J. Clin. Microbiol. 2012;50:953–960. doi: 10.1128/JCM.06179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Zhang Y., Yu M., Ren C., Gao Y., Yun B., Liu Y., Wang Y., Qi X., Liu C., Cui H., Zhang Y., Gao L., Li K., Pan Q., Zhang B., Wang X., Gao Y. Residues 28 to 39 of the Extracellular Loop 1 of chicken Na+/H+ Exchanger type I mediate cell binding and entry of subgroup J avian leukosis virus. J. Virol. 2018;92 doi: 10.1128/JVI.01627-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen S.L., Melder D.C., Federspiel M.J. Identification of key residues in subgroup A avian leukosis virus envelope Determining receptor binding Affinity and Infectivity of cells expressing chicken or Quail Tva receptor. J. Virol. 2001;75:726–737. doi: 10.1128/JVI.75.2.726-737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue D., Dambrine G., Denesvre C., Laurent S., Wyers M., Rasschaert D. Major rearrangements in the E element and minor variations in the U3 sequences of the avian leukosis subgroup J provirus isolated from field myelocytomatosis. Arch. Virol. 2006;151:2431–2446. doi: 10.1007/s00705-006-0811-2. [DOI] [PubMed] [Google Scholar]
- Ji X., Wang Q., Li X., Qi X., Wang Y., Gao H., Gao Y., Wang X. A 19-nucleotide insertion in the leader sequence of avian leukosis virus subgroup J contributes to its replication in vitro but is not related to its pathogenicity in vivo. PLoS One. 2014;9:e84797. doi: 10.1371/journal.pone.0084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R.A., Skalka A.M. A C-terminal domain in the avian sarcoma-leukosis virus pol gene product is not essential for viral replication. J. Virol. 1988;62:528–533. doi: 10.1128/jvi.62.2.528-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H., Zhang H., Ning Z., Chen R., Zhang W., Qing A., Xin C., Yu K., Cao W., Liao M. Isolation and characterization of emerging subgroup J avian leukosis virus associated with hemangioma in egg-type chickens. Vet. Microbiol. 2011;151:275–283. doi: 10.1016/j.vetmic.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Li T., Xie J., Liang G., Ren D., Sun S., Lv L., Xie Q., Shao H., Gao W., Qin A., Ye J. Co-infection of vvMDV with multiple subgroups of avian leukosis viruses in indigenous chicken flocks in China. BMC Vet. Res. 2019;15:288. doi: 10.1186/s12917-019-2041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounková A., Dráberová E., Šenigl F., Trejbalová K., Geryk J., Hejnar J., Svoboda J. Molecular events accompanying rous sarcoma virus rescue from rodent cells and the role of viral gene complementation. J. Virol. 2014;88:3505–3515. doi: 10.1128/JVI.02761-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounkova A., Kosla J., Prikryl D., Stafl K., Kucerova D., Svoboda J. Retroviral host range extension is coupled with Env-activating mutations resulting in receptor-independent entry. Proc. Natl. Acad. Sci. USA. 2017;114:E5148–E5157. doi: 10.1073/pnas.1704750114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei M., Ye J., Qin A., Wang L., Hu X., Qian K., Shao H. Identification of novel viral receptors with cell line expressing viral receptor-binding protein. Sci. Rep. 2015;5 doi: 10.1038/srep07935. 7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melder D.C., Pankratz V.S., Federspiel M.J. Evolutionary pressure of a receptor competitor selects different subgroup a avian leukosis virus escape variants with altered receptor interactions. J. Virol. 2003;77:10504–10514. doi: 10.1128/JVI.77.19.10504-10514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Li Q., Zhang Y., Zhang Z., Tian S., Cui Z., Chang S., Zhao P. Characterization of subgroup J avian Leukosis virus isolated from Chinese indigenous chickens. Virol. J. 2018;15:33. doi: 10.1186/s12985-018-0947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munguia A., Federspiel M.J. Avian sarcoma and leukosis virus envelope Glycoproteins Evolve to Broaden receptor usage under pressure from entry Competitors (dagger) Viruses. 2019;11:519. doi: 10.3390/v11060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V. Leukosis/Sarcoma Group. In: Swayne D.E., Boulianne M., Logue C.M., McDougald L.R., Nair V., Suarez D.L., editors. Diseases of Poultry. John Wiley & Sons, Inc; Hoboken, NJ: 2020. pp. 587–624. [Google Scholar]
- Nichol S. RNA viruses. Life on the edge of catastrophe. Nature. 1996;384:218–219. doi: 10.1038/384218a0. [DOI] [PubMed] [Google Scholar]
- Pan W., Gao Y., Qin L., Ni W., Liu Z., Yun B., Wang Y., Qi X., Gao H., Wang X. Genetic diversity and phylogenetic analysis of glycoprotein GP85 of ALV-J isolates from Mainland China between 1999 and 2010: coexistence of two extremely different subgroups in layers. Vet. Microbiol. 2012;156:205–212. doi: 10.1016/j.vetmic.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Payne L.N., Brown S.R., Bumstead N., Howes K., Frazier J.A., Thouless M.E. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 1991;72(Pt 4):801–807. doi: 10.1099/0022-1317-72-4-801. [DOI] [PubMed] [Google Scholar]
- Qin A., Lee L.F., Fadly A., Hunt H., Cui Z. Development and characterization of monoclonal antibodies to subgroup J avian leukosis virus. Avian Dis. 2001;45:938–945. [PubMed] [Google Scholar]
- Smith L.M., Brown S.R., Howes K., McLeod S., Arshad S.S., Barron G.S., Venugopal K., McKay J.C., Payne L.N. Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus. Virus Res. 1998;54:87–98. doi: 10.1016/s0168-1702(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Su Q., Li Y., Cui Z., Chang S., Zhao P. The emerging novel avian leukosis virus with mutations in the pol gene shows competitive replication advantages both in vivo and in vitro. Emerg. Microbes Infect. 2018;7:117. doi: 10.1038/s41426-018-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Graham W.D., Zhou S. Sequencing the Biology of entry: the retroviral env gene. In: Hunter E., Bister K., editors. Viruses, Genes, and Cancer. Springer International Publishing; Cham, Switzerland: 2017. pp. 65–82. [Google Scholar]
- Wang L., Mei M., Qin A., Ye J., Qian K., Shao H. Membrane-associated GRP78 helps subgroup J avian leucosis virus enter cells. Vet. Res. 2016;47:92. doi: 10.1186/s13567-016-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Qian K., Qin A., Shen H., Wang P., Jin W., Eltahir Y.M. Recombinant avian leukosis viruses of subgroup J isolated from field infected commercial layer chickens with hemangioma and myeloid leukosis possess an insertion in the E element. Vet. Res. Commun. 2010;34:619–632. doi: 10.1007/s11259-010-9436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Dong W., Yu C., He Z., Lv Y., Sun Y., Feng X., Li N., Lee L.F., Li M. Occurrence of avian leukosis virus subgroup J in commercial layer flocks in China. Avian Pathol. 2004;33:13–17. doi: 10.1080/03079450310001636237a. [DOI] [PubMed] [Google Scholar]
- Yao Y., Smith L.P., Nair V., Watson M.J. J.o.V. An avian Retrovirus Uses Canonical expression and Processing Mechanisms to Generate viral MicroRNA. J. Virol. 2014;88:2–9. doi: 10.1128/JVI.02921-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala G., Cheng S., Jackwood M.W. Molecular epidemiology of avian leukosis virus subgroup J and evolutionary history of its 3' untranslated region. Avian Dis. 2007;51:942–953. doi: 10.1637/0005-2086(2007)51[942:MEOALV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wu C.X., Chamba Y., Ling Y. Blood characteristics for high altitude adaptation in Tibetan chickens. Poult. Sci. 2007;86:1384–1389. doi: 10.1093/ps/86.7.1384. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Gou W., Wang X., Zhang Y., Ma J., Zhang H., Zhang Y., Zhang H. Genome Resequencing Identifies unique Adaptations of Tibetan chickens to Hypoxia and high-dose Ultraviolet Radiation in high-altitude environments. Genome Biol. Evol. 2016;8:765–776. doi: 10.1093/gbe/evw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang L., Shen A., Shen X., Xu M., Qian K., Shao H., Yao Y., Nair V., Ye J., Qin A. Detection of ALV p27 in cloacal swabs and virus isolation medium by sELISA. BMC Vet. Res. 2019;15:383. doi: 10.1186/s12917-019-2150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]