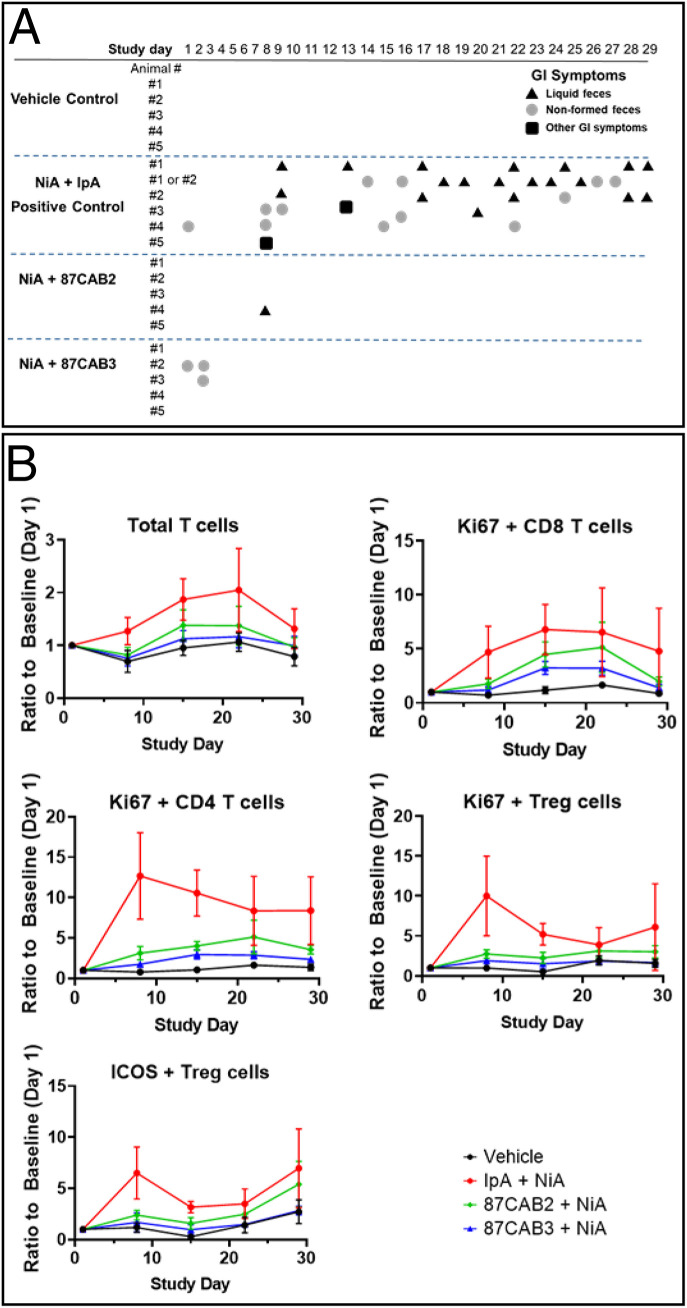
Fig. 4.
Anti-CTLA4 CABs nonhuman primate toxicity study. (A) Clinical observations of cynomolgus macaques treated in combination with anti-PD1 antibody (NiA) and anti-CTLA4 antibodies (IpA and CABs 87CAB2 and 87CAB3). Gastrointestinal toxicity was monitored as previously described (58) by measuring liquid feces or diarrhea (triangles), loosely formed feces (circles), or other GI symptoms such as vomiting or failure to eat food (squares). In some cases (animals 1 and 2), the source of liquid feces or loose stools could not be determined, as they were cohabitated during the experiment and listed as either 1 or 2. (B) Immunophenotyping of PBMC isolated from blood samples taken during the time course of anti-PD1 and anti-CTLA4 antibody treatments. Day 1 represents pretreatment baseline measurements, and day 29 represents 7 d following the last (fourth) antibody treatment. PBMC samples were isolated from heparinized blood samples by standard density gradient centrifugation using Ficoll−Hypaque medium. PBMCs were analyzed with antibodies that specifically recognize T cells (CD3) or T cell subsets T helper (CD4), T cytotoxic (CD8), or Treg cells (CD3, CD4, CD25, CD127, and FoxP3) as previously described (58). Cell activation state was measured by staining for the nuclear antigen Ki67. Inducible T cell costimulator (ICOS) staining was used as an additional antigen to also determine the level of the peripheral Treg cell activation state. The absolute levels and ratios of cells were compared by measuring the mean fluorescent intensity produced by staining using flow cytometry as previously described (58).