Summary
The development of DNA sequencing technology has provided an effective method for studying foodborne and phytopathogenic microorganisms on fruits and vegetables (F & V). DNA sequencing has successfully proceeded through three generations, including the tens of operating platforms. These advances have significantly promoted microbial whole‐genome sequencing (WGS) and DNA polymorphism research. Based on genomic and regional polymorphisms, genetic markers have been widely obtained. These molecular markers are used as targets for PCR or chip analyses to detect microbes at the genetic level. Furthermore, metagenomic analyses conducted by sequencing the hypervariable regions of ribosomal DNA (rDNA) have revealed comprehensive microbial communities in various studies on F & V. This review highlights the basic principles of three generations of DNA sequencing, and summarizes the WGS studies of and available DNA markers for major bacterial foodborne pathogens and phytopathogenic fungi found on F & V. In addition, rDNA sequencing‐based bacterial and fungal metagenomics are summarized under three topics. These findings deepen the understanding of DNA sequencing and its application in studies of foodborne and phytopathogenic microbes and shed light on strategies for the monitoring of F & V microbes and quality control.
The principles of three generations DNA sequencing were depicted. Whole genome sequencing and DNA markers of foodborne and plant fungal pathogens were summarized. Bacterial and fungal metagenomics studies on fruits and vegetables were concluded.
Introduction
The requirements for the improvement of the quality and safety of horticultural fruits and vegetables (F & V) depend on a better understanding of microorganisms (Dean et al., 2012; Olaimat and Holley, 2012; Siroli et al., 2015). Foodborne pathogens pollute F & V under cultivation in diverse environments, the use of unsterilized agricultural inputs or improper storage. Such contamination can easily cause food poisoning (Olaimat and Holley, 2012). Phytopathogenic fungi cause plant diseases, postharvest deterioration and mycotoxin accumulation, which significantly affect yield, quality and market value (Dean et al., 2012; Kumar et al., 2017). However, several endophytes can also be used as biocontrol agents to provide beneficial conditions for cultivation and postharvest storage (Siroli et al., 2015). Researchers are working to describe and control all of these microorganisms from farmland to consumers.
Microbial genome analysis relies strictly on DNA sequencing technology. The genome is the collection of DNA molecules, in which genes and variable sequences are arranged and provide the basic information for the formation of a microorganism. DNA sequencing, as a general technology applied in life science research, determines the nucleotide sequences of DNA strands. Over the past four decades, DNA sequencing technologies have rapidly developed and proceeded through three generations, resulting in the successful development of tens of platforms (Morey et al., 2013). First‐generation sequencing (FGS) was developed in the mid‐1970s and was mainly based on Frederick Sanger's DNA chain‐termination sequencing method (Sanger et al., 1974; Liu et al., 2012). Next‐generation sequencing (NGS) was introduced in the 2000s, involving systems such as the Roche 454 pyrosequencing, Illumina Genome Solexa and Supported Oligo Ligation Detection (SOLiD) platforms (Liu et al., 2012). Third‐generation sequencing (TGS) is the most recently introduced advance in this technology, which detects single and longer reads in real‐time with a high efficiency (van Dijk et al., 2018). In parallel, DNA sequencing technologies have been applied in various genomic and phylogenetic studies (Rogers et al., 2008; Morey et al., 2013). First, DNA sequencing and whole‐genome sequencing (WGS) were applied in microbial studies. Sanger sequencing was first used to determine the genome of phage X174 (5386 bp; Sanger et al., 1977). Subsequently, Sanger et al. verified the sequencing procedure and determined the genome of phage λ (48 502 bp; Sanger et al., 1983). In 1990, Goebel et al. reported the whole genome of vaccinia virus (192 kb), obtained by using the first automatic DNA sequencer, the AB370 system (Goebel et al., 1990). In 1991, Bankier et al. reported the genome of a human cytomegalovirus (229 kb; Bankier et al., 1991). In 1990, genomic research was initiated in Escherichia coli and Saccharomyces cerevisiae as model systems in preparation for the Human Genome Project. In 1995, Fleischmann et al. reported the genome of the first cellular microbe, Haemophilus influenzae Rd (1.83 Mb; Fleischmann et al., 1995). After the AB370 DNA sequencer was upgraded to the AB3730xl system, microbial WGS was greatly promoted. Since then, the genomes of important microbes such as E. coli (Blattner et al., 1997), Salmonella enterica (Parkhill et al., 2001), Listeria monocytogenes (Glaser et al., 2001), Staphylococcus aureus (Kuroda et al., 2001), Campylobacter jejuni (Parkhill et al., 2000) and Shigella flexneri (Jin et al., 2002) have been widely reported. Currently, approximately 100 microbial genomes per day are being registered on the US National Center for Biotechnology Information (NCBI) platform.
Knowledge of DNA polymorphisms improves the understanding of microbial genetic specificity. The microbial genome shows various sequence differences or polymorphisms. Microbial DNA polymorphisms are the basis for explaining the specificity of phenotypes, evolution and taxonomy (Foley et al., 2009). Methods such as the amplified fragment length polymorphism (AFLP), random fragment length polymorphism (RFLP), randomly amplified polymorphic DNA (RAPD), simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) approaches are important for DNA polymorphism studies. Significantly, polymorphisms in bacterial ribosomal DNA (rDNA) 16S rRNA genes and fungal internal transcribed spacers (ITSs) have been widely used in studies of microbial taxonomy and identification (Sun et al., 2013). The 16S rDNA sequences of bacteria are relatively short, containing several conserved and hypervariable regions, and can provide taxonomic information for bacteria at the genetic level. Fungal rDNA contains tandem repeats of noncoding ITS regions. These ITS regions show a high level of polymorphism, and they are effective for fungal identification. Recently, improved sequencing technologies and powerful databases and software have promoted the development of sequence‐based identification methods. In metagenomics, 16S rDNA and ITS sequencing present significant benefits for characterizing overall bacterial and fungal communities.
Notably, DNA sequencing has promoted studies involving F & V microbial WGS, gene identification and specificity analysis. Genetic markers identified in studies of polymorphism have been widely used for polymerase chain reaction‐ (PCR) and chip‐based microbial detection (based, e.g. on conventional PCR, qPCR, multiplex PCR and gene chips; Lüth et al., 2018). These detection methods can monitor foodborne pathogens and phytopathogens on F & V with good accuracy and acceptability (O'Connor and Glynn, 2010). In addition, metagenomic studies have revealed the bacterial and fungal communities on F & V by using 16S rDNA and ITS sequencing (Forbes et al., 2017). The present review highlights the principles of DNA sequencing and outlines the WGS information and genetic markers of major bacterial foodborne pathogens and fungal phytopathogens on F & V. Common foodborne pathogenic species come from the Escherichia, Salmonella, Staphylococcus, Listeria, Shigella and Campylobacter genera (Table 1). Common phytopathogenic fungi come from the Penicillium, Alternaria, Aspergillus, Fusarium, Botrytis, Colletotrichum, Monilinia and Trichothecium genera (Table 2). Furthermore, NGS‐based metagenomic references have provided comprehensive data on key aspects of bacterial and fungal communities found on F & V (Table 3). These findings have deepened our understanding of DNA sequencing technology and its application in studies of foodborne and phytopathogenic pathogens, and they have shed light on methods for the microbial monitoring and quality control of F & V.
Table 1.
Whole‐genome sequences of foodborne pathogens registered on NCBI platforms (up to 1 October 2019) and summary of the related genetic markers.
| Species | ID number | Number of sequenced strains | Representative strain | Size (Mb) | GC% | Genes | Proteins | Specific genetic markers |
|---|---|---|---|---|---|---|---|---|
| Escherichia | ||||||||
| E. coli | ID167 | 17 952 | K‐12 substr. MG1655 (Blattner et al., 1997) | 4.64 | 50.8 | 4498 | 4140 | fliC, Vt1, Vt2 (Gannon et al., 1997); uspA (Osek, 2001); lacZ (Foulds et al., 2002); rfbE, eae, stx1, stx2 (Ooka et al., 2009); ipaH (van den Beld and Reubsaet, 2012); lacY, uidA (Mendes Silva and Domingues, 2015); PhoA (Yang et al., 2016); cdtB (Hassan et al., 2018) |
| E. albertii | ID 1729 | 89 | KF1 | 4.70 | 49.7 | 4823 | 4380 | cdtB (Maheux et al., 2014); rpoB (Lindsey et al., 2015); EAKF1_ch4033 (Lindsey et al., 2017); 16S rDNA (Grillova et al., 2018) |
| E. fergusonii | ID 1877 | 18 | ATCC 35469T | 4.64 | 49.9 | 4618 | 4349 | yliE, EFER_1569, EFER_3126 (Simmons et al., 2014); EFER_0790 (Lindsey et al., 2017) |
| Salmonella | ||||||||
| S. enterica | ID 152 | 11 215 | CT18 | 5.13 | 51.9 | 4829 | 4473 | AceK, fliC, iagA, invA, oriC, sdf, sefA, ssaN, ssrA, STM2745, STM4492, 16S rRNA (Lee et al., 2009; Postollec et al., 2011); sseL, spvC (Peterson et al., 2010); hilA, fimA, hns (Jeyasekaran et al., 2011); avrA, stn, stm (Amin et al., 2016); spv, hut, fljB (Alzwghaibi et al., 2018) |
| S. bongori | ID1089 | 20 | NCTC 12419 | 4.46 | 51.3 | 4382 | 4068 | fljB, gatD, invA (Lee et al., 2009); fliC, gnd, mutS (Soler‐Garcia et al., 2014) |
| Staphylococcus | ||||||||
| S. aureus | ID 154 | 10 630 | NCTC 8325 | 2.82 | 32.9 | 2872 | 2767 | mecA, nuc, femA‐SA, femA‐SE, orfx‐SCCmec, spa, gyrB, 16S rRNA (Hirvonen, 2014); tuf, rpoB, gap, pyrH, ftsZ (Song et al., 2019); sea, seb, sec, sed, see (Omwenga et al., 2019) |
| S. epidermidis | ID 155 | 670 | ATCC 12228 | 2.56 | 32.1 | 2558 | 2482 | mecA, ermA, ermB, ermC, and msrA (Martineau et al., 2000); hld; ica, agrA, sarA (Frebourg et al., 2000); 16S rDNA and tuf (Kobayashi et al., 2009); femA‐SA, femA‐SE (Jukes et al., 2010); atlE, gap and mvaA (Kilic and Basustaoglu, 2011); recN (Iorio et al., 2011); adhesin fibrinogen binding protein (Sunagar et al., 2013); hla/yidD and hlb (Pinheiro et al., 2015); Gmk2, pta and SESB (Osmani Bojd et al., 2017); icaA, aap, bhp (Ribic et al., 2017) |
| S. lugdunensis | ID2548 | 26 | HKU09‐01 | 2.66 | 33.9 | 2567 | 2567 | Agr (Dufour et al., 2002); rpoB (Drancourt and Raoult, 2002); 16S rRNA (Skow et al., 2005); Fbl (Campos‐Pena et al., 2014) |
| S. saprophyticus | ID1350 | 108 | ATCC 15305 | 2.58 | 33.1 | 2523 | 2351 | 16S rRNA (Gaszewska‐Mastalarz et al., 1997); femA (Vannuffel et al., 1999); hrcA (Paiva‐Santos et al., 2016) |
| S. pseudintermedius | ID3429 | 223 | HKU10‐03 | 2.62 | 37.5 | 2517 | 2384 | SpsJ (Verstappen et al., 2017); sps (Phumthanakorn et al., 2017) |
| Listeria | ||||||||
| L. monocytogenes | ID 159 | 3063 | EGD‐e | 2.94 | 38.0 | 3055 | 2867 | hly, iap, mpl, prfA, inlA, inlB, actA (Gasanov et al., 2005); plcA, 16S RNA (Xu et al., 2008) |
| L. seeligeri | ID 1246 | 6 | SLCC3954 | 2.80 | 37.4 | 2790 | 2663 | 23S rRNA, iap (Paillard et al., 2003); lse24‐315 (Liu et al., 2004); 16S rRNA (Dalmasso et al., 2010); 5'‐exonuclease (Hage et al., 2014) |
| Shigella | ||||||||
| S. flexneri | ID 182 | 480 | 301 | 4.83 | 50.67 | 4788 | 4313 | ipaH, plasmid DNA, ial, 16S rRNA (Warren et al., 2006); she PAI (Farfan et al., 2010); invC, rfc (Ojha et al., 2013); genome marker (Sahl et al., 2015; Kim et al., 2017a) |
| S. boydii | ID 496 | 113 | 1221_SBOY | 4.84 | 50.7 | 5125 | 4745 | ipaH, 16S rRNA (Warren et al., 2006); invC (Ojha et al., 2013); wzy (Radhika et al., 2014); genome marker (Sahl et al., 2015; Kim et al., 2017a) |
| S. sonnei | ID 417 | 1338 | 4303 | 4.55 | 51.1 | 5275 | 4009 | ipaH, 16S rRNA (Warren et al., 2006); IS1 (Hsu et al., 2007); she PAI (Farfan et al., 2010); invC, wbgZ (Ojha et al., 2013); IpaBCD (Farshad et al., 2015); genome marker (Sahl et al., 2015; Kim et al., 2017a) |
| S. dysenteriae | ID 415 | 67 | Sd197 | 4.56 | 50.9 | 4834 | 4294 | ial, virA, 16S rRNA (Warren et al., 2006); invC, rfpB (Ojha et al., 2013); genome marker (Sahl et al., 2015; Kim et al., 2017a) |
| Campylobacter | ||||||||
| C. jejuni | ID149 | 1615 | NCTC 11168 | 1.64 | 30.5 | 1668 | 1572 | pDT1720 (Ng et al., 1997); CC2, CJ2 (Sails et al., 2001); ciaB, pldA, CDT (Ghorbanalizadgan et al., 2014); GTPase gene, hip, 16S rRNA, rrs, cdaF, porA, 16S‐23S ITS, Hyp, cjaA, ceuE, hipO, mapA, ceuA, askD, glyA, lpxA, ccoN, ORF‐C sequence, rpoB, oxidoreductase gene, cdtA, pepT (Frasao et al., 2017); flaA, cadF, racR, dnaJ, cdtB, and cdtC (Oh et al., 2017); gyrA (Sierra‐Arguello et al., 2018); ask, cdt (Kabir et al., 2019) |
| C. coli | ID1145 | 928 | Aerotolerant OR12 | 2.03 | 30.8 | 2185 | 2058 | arsP, arsR, arsC, acr3, arsB (Noormohamed and Fakhr, 2013); GTPase gene, hip, ceuA, CCCH, cdtB, porA, 16S‐23S ITS, ceuE, mapA, hipO, glyA, cadF, oxidoreductase gene, cdtA, pepT, 50S gene, VS1 (Frasao et al., 2017); ceuE (Rodgers et al., 2017); cadF, asp (Pavlova et al., 2016; Banowary et al., 2018) |
Table 2.
Whole‐genome sequencing of phytopathogenic fungi registered on NCBI platforms (up to 1 October 2019) and summary of the related genetic markers.
| Species | ID number | Number of sequenced strains | Representative strain | Size (Mb) | GC% | Genes | Proteins | Specific genetic markers |
|---|---|---|---|---|---|---|---|---|
| Penicillium | ||||||||
| P. expansum | ID11336 | 9 | MD‐8 | 32.36 | 47.5 | 11 060 | 11 060 | Polygalacturonase (Hesham et al., 2011); PatF (Tannous et al., 2015); idh, 18S, β‐tubulin, calmodulin (De et al., 2016; Rharmitt et al., 2016); ITS1‐ITS4 (Hammami et al., 2017) |
| P. digitatum | ID13384 | 3 | Pd1 | 26.05 | 48.9 | 8962 | 8961 | PdCYP51 (Hamamoto et al., 2001); β‐tubulin (Oshikata et al., 2013); Pdcyt b (Zhang et al., 2009); ITS (Liu et al., 2017); RPB1, cmd (Chen et al., 2017b) |
| P. griseofulvum | ID43141 | 2 | PG3 | 29.14 | 47.3 | 9630 | 9630 | ITS and IAO (Shi et al., 2011) |
| P. citrinum | ID40785 | 2 | JCM 22607 | 34.00 | 45.9 | – | – | msdC (Yoshida and Ichishima, 1995); ITS (Song et al., 2018) |
| P. italicum | ID34360 | 3 | GL‐Gan1 | 31.03 | 45.8 | – | – | ITS (Youssef et al., 2010); RPB1‐1, cmd‐3 (Chen et al., 2017b); RPB1, RPB2 (Chen et al., 2019) |
| Alternaria | ||||||||
| A. alternata | ID11201 | 6 | SRC1lrK2f | 32.99 | 51.4 | 13 577 | 13 466 | endopolygalacturonase (Garganese et al., 2016); glyceraldehyde 3‐phosphate dehydrogenase (Gpd), Alt a1 (Gherbawy et al., 2018); AaSdhB, AaSdhC, AaSdhD (Lichtemberg et al., 2018); β‐tubulin (Basim et al., 2018); ITS1, ITS4, histone 3 (Wang et al., 2019) |
| A. arborescens | ID12091 | 5 | EGS 39‐128 | 33.89 | 50.9 | – | – | ITS (Lorenzini and Zapparoli, 2014); endopolygalacturonase (Garganese et al., 2016); Alt a1, calmodulin, plasma membrane ATPase (Elfar et al., 2018); elongation factor, β‐tubulin, glyceraldehyde‐3‐phosphate dehydrogenase (Somma et al., 2019) |
| A. brassicicola | ID865 | 2 | Abra43 | 31.04 | 50.8 | – | – | Cutinase A (Gachon and Saindrenan, 2004); Microsatellite locus ABS28 (Singh et al., 2014); ITS4, ITS5 (Gao et al., 2014) |
| A. solani | ID65961 | 2 | NL03003 | 32.78 | 51.3 | – | – | ITS1, ITS2 (Zur et al., 2002); Alt_a1, ITS‐ptAs (Gu et al., 2017); histidine kinase (HK1) (Khan et al., 2018) |
| A. tenuissima | ID76065 | 7 | FERA 1166 | 35.70 | 51.1 | 13 653 | 13 575 | ITS1, ITS4 (You et al., 2014); AM‐toxin (Andersen et al., 2006); histone (Kou et al., 2014) |
| Aspergillus | ||||||||
| A. flavus | ID360 | 60 | NRRL3357 | 36.89 | 48.3 | 13 485 | 13 485 | aflS, aflO (Degola et al., 2007); ITS, pksA, omtA (Yin et al., 2009b); β‐tubulin (Zarrinfar et al., 2015); aflatoxin biosynthesis gene cluster (Callicott and Cotty, 2015); aflQ (Mahmoud, 2015); ITS1, ITS4 (Mylroie et al., 2016); aflR –aflJ intergenic region (Atoui and El Khoury, 2017); ITS, aflP(1), aflM, aflA, aflD, aflP(3), aflP(2), aflR (Al‐Shuhaib et al., 2018); norA, omtA (Hua et al., 2018) |
| A. niger | ID429 | 14 | FDAARGOS_311 | 35.74 | 49.7 | 11 602 | 11 190 | ITS (Gonzalez‐Salgado et al., 2005); aclF1 (Tuntevski et al., 2013); β‐tubulin gene (Mirhendi et al., 2016); calmodulin gene (Das et al., 2017); benA (von Hertwig et al., 2018) |
| A. parasiticus | ID12976 | 3 | SU‐1 | 39.47 | 48.1 | 8645 | 8645 | nor‐1, ver‐1, omt‐1, apa‐2 (Chen et al., 2002); aflR (Somashekar et al., 2004); ITS (Luo et al., 2009); aflC, norA (Yin et al., 2015); aflR‐aflJ intergenic region (Atoui and El Khoury, 2017) |
| A. tubingensis | ID18109 | 2 | CBS 134.48 | 35.15 | 49.2 | 12 592 | 12 319 | ITS (Medina et al., 2005); sequence‐characterized amplified region (SCAR) (Giaj Merlera et al., 2015); calmodulin (Palumbo and O'Keeffe, 2015); β‐tubulin (Mirhendi et al., 2016) |
| A. carbonarius | ID947 | 1 | ITEM 5010 | 36.15 | 51.6 | 11 735 | 11 478 | Calmodulin (Palumbo and O'Keeffe, 2015); ITS (Tryfinopoulou et al., 2015); acOTApks, acOTAnrps, acpks, laeA, veA (El Khoury et al., 2016) |
| A. westerdijkiae | ID40399 | 1 | CBS 112803 (Abdel‐Wahhab et al., 2016) | 36.07 | 50.2 | – | – | ITS (Gil‐Serna et al., 2009); pks, p450‐B03 (Gil‐Serna et al., 2011); β‐tubulin (Durand et al., 2019) |
| Fusarium | ||||||||
| F. oxysporum | ID707 | 115 | f. sp. lycopersici 4287 (Ma et al., 2010) | 61.39 | 48.4 | 27 347 | 27 347 | FOW1 (Li et al., 2014); IGS, TEF‐1α, β‐tubulin gene (Kim et al., 2017b); Tfo1 insertion (Ortiz et al., 2017); Bik (Pugliese et al., 2013); ITS (Wu et al., 2019); Ef1α (Chen et al., 2017a); SIX4, SIX5 (Ayukawa et al., 2016); SIX1, SIX3 (Debbi et al., 2018); FEM1, HPEG (van Dam et al., 2018); FocScF/FocScR (Singh and Kapoor, 2018) |
| F. solani (Nectria haematococca) | ID 537 | 3 | 77‐13‐4 (Coleman et al., 2009) | 51.29 | 50.8 | 15 708 | 15 708 | FS1/FS2 (Casasnovas et al., 2013); EF‐1α (Muraosa et al., 2014); ITS (de Souza et al., 2017); RPB (Chitrampalam et al., 2018) |
| F. fujikuroi | ID 13188 | 15 | IMI 58289 (Wiemann et al., 2013) | 43.83 | 47.5 | 14 943 | 14 810 | histone H3 (Steenkamp et al., 1999); MAT allele (Steenkamp et al., 2000); ITS (Llorens et al., 2006); gaoB (Faria et al., 2012); β2‐tubulin (Zhang et al., 2015); TEF 1‐α (Carneiro et al., 2017) |
| F. verticillioides | ID 488 | 4 | 7600 (Ma et al., 2010) | 41.84 | 48.7 | 20 574 | 20 574 | EF‐1α (Wu et al., 2016); FUM1, FUM19 (Omori et al., 2018); ITS (Jedidi et al., 2018); calmodulin (Mulè et al., 2004); EGFP (Gai et al., 2018) |
| F. proliferatum | ID 2434 | 13 | ET1 (Niehaus et al., 2016) | 45.21 | 48.1 | 16 509 | 16 143 | Calmodulin (Mulè et al., 2004); ISR (Borrego‐Benjumea et al., 2014); SSR (Moncrief et al., 2016); tef‐1α, FUM1 (Galvez et al., 2017); FUM19 (Proctor and Vaughan, 2017); ITS (Jedidi et al., 2018) |
| F. graminearum | ID 58 | 11 | PH‐1 (Cuomo et al., 2007) | 36.67 | 48.3 | 13 334 | 13 334 | MGB (Waalwijk et al., 2004); gaoA (de Biazio et al., 2008); cyp51A, beta‐tubulin (Yin et al., 2009a); TRI12 (Nielsen et al., 2012); MAT (Demeke et al., 2010); PKS13 (Atoui et al., 2012); Tri7, Tri13 (Tralamazza et al., 2016); TRI (Wang and Cheng, 2017); ITS (Jedidi et al., 2018); tef‐1α (Garmendia et al., 2018) |
| Colletotrichum | ||||||||
| C. acutatum | ID38530 | 2 | 1 | 52.13 | 51.7 | – | – | ITS (Talhinhas et al., 2002); beta‐tubulin 2 (Talhinhas et al., 2005); CaInt2 (Jelev et al., 2008); BenA (Polizzi et al., 2011); beta‐tubulin (TUB), GAPDH (Karimi et al., 2019); MAT1‐2 (Furuta et al., 2017) |
| C. gloeosporioides | ID17739 | 6 | TYU | 53.01 | 49.6 | – | – | RAPD (Pileggi et al., 2009); CgInt (Lopes et al., 2010); β‐tubulin (TUB), actin (ACT), GPDH (Ramdeen and Rampersad, 2013); act, cal, gapdh, gs, ITS, tub2 (Sharma et al., 2017) |
| C. coccodes | ID56076 | 2 | NJ‐RT1 | 50.12 | 53.8 | – | – | RAPD (Dauch et al., 2003); ITS (Baysal‐Gurel et al., 2014); CAL, ITS, TUB2 (Silva et al., 2018) |
| C. fructicola | ID57524 | 3 | 15060 (Aguilar‐Pontes et al., 2018) | 55.92 | 53.2 | – | – | ITS (Li et al., 2013); ACT, CAL, CHS‐1, ITS, TUB, GAPDH (Gan et al., 2017) |
| Monilinia | ||||||||
| M. fructicola | ID54919 | 2 | LMK 125 | 44.68 | 40.1 | – | – | MO368‐1, Laxa (Cote et al., 2004); 18S rDNA (Fulton and Brown, 1997); β‐tubulin (Fan et al., 2014); ITS (Guinet et al., 2016); laccase‐2 (Wang et al., 2018b); cytochrome b (Ortega et al., 2019) |
| M. laxa | ID56076 | 2 | EBR‐Ba11b | 41.84 | 40.2 | – | – | 18S rDNA (Fulton and Brown, 1997); MO368‐1, Laxa (Cote et al., 2004); ISSR and RAPD (Fazekas et al., 2014); ITS (Guinet et al., 2016); laccase‐2 (Wang et al., 2018b); SCAR (Ortega et al., 2019) |
| M. fructigena | ID66926 | 3 | ASM290963v1 | 39.33 | 41.7 | – | – | 18S rDNA (Fulton and Brown, 1997); MO368‐1, Laxa (Cote et al., 2004); β‐tubulin (Fan et al., 2014); ITS (Guinet et al., 2016); laccase‐2 (Wang et al., 2018b) |
| M. polystroma | ID66925 | 1 | ASM290964v1 | 44.63 | 39.2 | – | – | MO368‐1, Laxa (Cote et al., 2004); ITS (Guinet et al., 2016); laccase‐2 (Wang et al., 2018b) |
| Botrytis | ||||||||
| B. cinerea | ID494 | 4 | B05.10 | 42.63 | 42.0 | 13 703 | 13 703 | EcoRI (Rigotti et al., 2002); IGS, SCAR (Suarez et al., 2005); IGS (Diguta et al., 2010); G3PDH, HSP60, RPB2, NEP1, NEP2 (Fan et al., 2015); ITS, β‐tubulin (Reich et al., 2016); necrosis, nep1 (Munoz et al., 2016) |
Table 3.
Summary of 16S rDNA and ITS sequencing‐based microbiome community studies of fruits and vegetables.
| Samples | Bacteria/fungi | Target organs | Study focus | Technology/sequencing platform | 16S rRNA target/primers | Taxonomic resolution/focused taxonomy | References |
|---|---|---|---|---|---|---|---|
| Topics | Microbiomes differences between plant species/genotypes | ||||||
| Apple | Bacteria | Flower | Apple flower Microbiome | Pyrosequencing | Primers 799 and 1115 reverse | Genera: Acetobacter; Arthrobacter; Burkholderia; Buttiauxella; Deinococcus; Escherichia/Shigella; Hymenobacter; Knoellia; Lactobacillus; Methanosarcina; Pantoea; Terrimonas; Truepera | Shade et al. (2013) |
| Apple, Grapes, Lettuce, Peach, Spinach and Tomato | Bacteria | Fruit; Leaf | Overall diversity and composition of bacterial communities, and their variations in fruits and vegetables | Pyrosequencing | Metabarcoding V4eV7 (universal primer 799F and 1115R) | Family: Bacillaceae; Comamonadaceae; Enterobacteriaceae; Flavobacteriaceae; Leuconostocaceae; Microbacteriaceae; Micrococcaceae; Moraxellaceae; Nocadioidaceae; Oxalobacteraceae; Pseudomonadaceae; Rhizobiaceae; Sphingomonadaceae; Xanthomonadaceae | Leff and Fierer (2013) |
| Arugula | Bacteria | Leaf | Arugula phyllosphere indigenous microbiome | Pyrosequencing | V3‐V8 | Genera: Caulobacterales; Rhizobaiales; Rhodobacterales; Sphingomonadales; Burkholderiales; Rhodocyclales; Enterobacteriales; Pseudomonadales; Xanthomonadales | Cernava et al. (2019) |
| Basil | Bacteria | Leaf | Naturally bacterial community on basil leaves | Pyrosequencing | V1‐V3 | Genera: Arcicella; Chryseobacterium; Flavobacterium; Sphingobacterium; Altererythrobacter; Novosphingobium; Sphingobium; Sphingomonas; Herbaspirillum; Acinetobacter; Pseudomonas; Rheinheimera; Enterobacter; Erwinia; Klebsiella; Kluyvera; Pantoea; Rahnella; Raoultella | Ceuppens et al. (2015) |
| Blueberry | Bacteria | Root | Soil properties and plant cultivars cooperatively shaped the variations in bacterial diversity and networks in the rhizosphere | Illumina sequencing | V4‐V5 | Genera: Azospirillum; Phenylobacterium; Rhodanobacter; Steroidobacter; Pseudomonas; Leteibacter; Acidothermus; Dongia; Rhizomicrobium; Pseudolabrys; Acidothermus; Rhodanobacter; Bradyrhizobium; Rhodoplanes; Pseudolabrys | Jiang et al. (2017) |
| Cilantro, Cucumber, Mung, Bean and Sprout | Bacteria | Fruit, Leaf and Stem | Bacterial diversity differences | Illumina sequencing | V1‐V3 | Genera: Xanthomonas; Sphingobacterium; Stenotrophomonas; Klebsiella; Enterococcus; Corynebacterium; Braachybacterium; Cronobacter; Brevibacterium; Rhizobium; Weissella; Serratia; Acinetobacter; Pantoea; Pseudomonas; Enterobacteriaceae; Bacillus; Enterobacter; Staphylococcus | Jarvis et al. (2018) |
| Grape | Bacteria | Leaf | Characterize the natural microbiome of grapevine | Pyrosequencing | V6 | Genera: Neisseriaceae; Sphingomonadaceae; Xanthomonadaceae; Veillonellaceae; Comamonadaceae; Leuconostocaceae; Moraxellaceae; Pseudomonadaceae; Enterobacteriaceae; Streptococcaceae | Pinto et al. (2014) |
| Grape | Bacteria | Fruit | Geographical origin and cultivar of grape by microbiome composition in Northern Italy and Spain Vineyards | Illumina sequencing | V3‐V4 | Genera: Alphaproteobacteria; Gamaproteobacteria; Actinobacteria; Bacilli; Clostridia; Shingobactellia; Cytophagia; Blastocatellia; Phycisphaerae; Acidimicrobia; Thermoleophilia; Deltaproteobacteria; Solibacteres; Gemmatimonadetes; Actinobacteria; Cyanobacteria | Mezzasalma et al. (2018) |
| Grape | Bacteria | Fruit | Bacterial communities of Grenache and Carignan grape varieties | Not given | V4 | Genera: Bacillus; Oenococcus; Acinetobacter; Erwinia; Streptococcus; Pseudomonas; Haemophilus; Enhydrobacter; Methylobacterium; Veillonella; Corynebacterium; Lactobacillus; Neisseria; Gluconobacter; Sphingomonas; Staphylococcus; Micrococcus | Portillo et al. (2016) |
| Grape | Fungi | Fruit; Leaf | Microbial relationship between soil, leaves and fruits | Illumina sequencing | ITS1 | Genera: Hydropisphaera; Mycoarthris; Chaetomium; Fusarium; Acremonium; Phoma; Cryptococcus; Cladosporium; Guehomyces; Alternaria | Zhang et al. (2017b) |
| Grape | Fungi | Leaf | Natural microbiome of grapevine | Pyrosequencing | ITS2 and D2 | Genera: Zoophthora; Pandora; Rhizopus; Mucor; Aureobasidiu; Sporormiella; Alternaria; Kurtzmanomyces; Colacogloea, Lewia; Ustilago; Puccinia; Cronartium | Pinto et al. (2014) |
| Grape | Fungi | Fruit | Wine grape yeast from China | Not given | ITS1 | Genera: Zygosaccharomyces; Candida; Issatchenkia; Pichia; Sporidiobolus; Hanseniaspora; Cryptococcus; Pichia; Issatchenkia; Metschnikowia | Li et al. (2010) |
| Grape | Bacteria | Leaf; Fruit | Microbial relationship between soil, leaves and grapes | Illumina sequencing | V5‐V7 | Genera: Kocuria; Microvirga; Flavobacterium; Sphingomonas; Nocardioides; Pseudomonas; Gaiella; Arthrobacter; Bacillus; Blastococcus | Zhang et al. (2017b) |
| Grape | Bacteria | Fruit | Geography and cultivar differences affect wine grape microbiomes | Illumina sequencing | V4 | Genera: Acetobacter; Erwinia; Gluconobacter; Hymenobacter; Janthinobacterium; Klebsiella; Lactobacillus; Microbacteriaceae; Sporosarcina; Pseudomonadaceae; Sphingomonas; Leuconostocaceae; Moraxellaceae; Methylobacterium | Bokulich et al. (2014) |
| Grape | Fungi | Fruit | Geography and cultivar differences affect wine grape microbiomes | Illumina sequencing | ITS1 | Genera: Cladosporium spp.; Botryotinia Fuckeliana, Penicillium; Davidiella; Aureobasidium; Hanseniaspora; Candida | Bokulich et al. (2014) |
| Grape | Fungi | Fruit | Cultivar differences affect microbial communities | Illumina sequencing | ITS1 | Genera: Myrothecium; Epicoccum; Cryptococcus; Mortierella; Guehomyces; Fusanrium; Phoma; Cladosporium; Alternaria | Zhang et al. (2017a) |
| Kiwifruit | Bacteria | Leaf and Flower | Plant species, organ and Psa infection in shaping bacterial phyllosphere communities | Illumina sequencing | V3‐V4 | Genera: Acinetobacter; Actinobacteria; Bacillus; Brevundimonae; Massillia; Methylobacterium; Pseudomonas; Sphingomonas; Streptococcus | Purahong et al. (2018) |
| Spinach and Rocket | Bacteria | Leaf | Leaf mineral content related to microbial community structure | Illumina sequencing | V3 | Genera: Aeromonas; Bacillus; Citrobacter; Curtobacterium; Enterobacter; Lelliottia; Obesumbacterium; Pantoea; Providencia; Pseudomonas; Shigella | Darlison et al. (2019) |
| Spinach and Rocket | Fungi | Leaf | Leaf mineral content related to microbial community structure | Illumina sequencing | V3 | Genera: Dothioraceae; Davidiellaceae; Tremellales; Incertae Sedis; Davidiellaceae; Cystofilobasidiaceae; Tremellales | Chen et al. (2018) |
| Tomato | Bacteria | Flower, Fruit, Leaf, Root and Stem | Tomato microbial diversity reflect the ecology of pathogenicity | Pyrosequencing | V2 | Genera: Pseudomonas; Micrococcineae; Xanthomonas; MethyloBacterium; Rhizobium; Sphingomonas | Ottesen et al. (2013) |
| Tomato | Fungi | Flower, Fruit, Leaf, Root and Stem | Tomato microbial diversity reflect the ecology of pathogenicity | Pyrosequencing | EF4 and Fung5 | Genera: Hypocrea; Aureobasidium; Cryptococcus; Chaetomium; Fusarium; Aspergillus | Ottesen et al. (2013) |
| Topics | Regional environment and farming practices affect microbiomes | ||||||
|---|---|---|---|---|---|---|---|
| Apple | Fungi | Fruit | Location‐related fungal differences reflect microbial quality | Illumina sequencing | ITS1 | Genera: Stibella; Paraphaeosphaeria; Phoma; Tilletiopsis; Cryptococcus; Aureobasidium; Acremonium | Shen et al. (2018b) |
| Cabbage and Lettuce | Bacteria | Leaf | Microbial communities location differences | Pyrosequencing | V4‐V5 | Genera: Acinetobacter; Alistipes; Barnesiella; Rikenella; Anaerococcus; Aerosphaera; Vagococcus; Parabacteroides; Kluyvera; Peptoclostriduim; Lysinibacillus; Lactobacillus; Serratia; Macrococcus; Weisella; Exiguobacterium; Kosakonia; Candidatus Nardonella; Leclercia; Obesumbacterium; Streptococcus; Escherichia; Clostridium; Aeromonas; Staphylococcus; Lactococcus; Buttiauxella; Citrobacter; Pseudomonas; Enterococcus; Klebsiella; Bacillus; Enterobacter; Pantoea | Higgins et al. (2018) |
| Grape | Fungi | Fruit | Fungal diversity in ferments associated with grape microbes | Pyrosequencing | NL1 and NL4 | Genera: Columnosphaeria; Davidiella; Cryptococcus | Morrison‐Whittle et al. (2017) |
| Grape | Fungi | Fruit | Grape microbiome related to field origin and environmental conditions | Illumina sequencing | ITS1 | Family: Botryosphaeriaceae; Dothioraceae; Pleosporaceae; Trichocomaceae; Incertae Sedis; Saccharomycetaceae; Saccharomycodaceae; Hymenochaetales; Peniophoraceae; Chytridiomycota | Mezzasalma et al. (2017) |
| Lettuce | Bacteria | Leaf | Bacterial communities on plant leaf surfaces related to time, space and environment | Pyrosequencing | V5‐V7 | Genera: Xanthomonas; Pantoea; Pseudomonas; Massilla; Bacillus; Erwinia; Alkanindiges; Acinetobacter; Naxibacter; Duganella; Flavobacterium; Exiguobacterium; Arthrobacter | Rastogi et al. (2012) |
| Lettuce | Bacteria | Leaf | Biotic stress shifted structure and abundance of Enterobacteriaceae in the lettuce microbiome | Pyrosequencing | V4‐V5 | Genera: Trabulsiella; Serratia; Klubsiella; Yersinia; Rahnella; Pantoea; Escherichia; Enterobacter; Buttiauxella; Cedecea; Citrobacter; Erwinia | Erlacher et al. (2015) |
| Maize | Bacteria | Leaf | Organic fertilization increased antibiotic resistome in phyllosphere | Illumina sequencing | V4‐V5 | Genera: Sphingomonas; Pseudomonas; Chryseobacterium; Curtobacterium; Methylobacterium | Chen et al. (2018) |
| Mulberry | Fungi | Fruit | Soil fungal community borne related to genotypes and disease occurrence | Illumina sequencing | ITS1 and ITS2 | Genera: Humicola; Mortierella; Scleromitrula; Schizothecium; Sphaerulina; Lecidella; Cortinarius; Russula; Hymenochaete; Mrakia | Yu et al. (2016) |
| Peach and Nectarines | Fungi | Fruit | Yeasts traceability of organic and conventional farming types | PCR‐DGGE/GATC Biotech | D1/D2 | Species: Pichia Kluyveri; Pichia Fermentans; Candida; Tremella Flava | Bigot et al. (2015) |
| Tomato | Bacteria | Flower and Fruit | Insects introduce variability in bacterial diversity | Illumina sequencing | V1‐V3 | Family: Microbacteriaceae; Methylobacteriaceae; Rhizobiales; Alphaproteobacteria; Proteobacteria; Rhizobiaceae; Sphingomonadaceae; Comamonadaceae; Gammaproteobacteria; Shewanellaceae; Enterobacteriaceae; Pseudomonadales; Pseudomonadaceae; Xanthomonadaceae | Allard et al. (2018) |
| Topics | Artificial treatment and quality control in storage and processing | ||||||
|---|---|---|---|---|---|---|---|
| Apple | Fungi | Fruit | Long‐term cold storage changed fungal components | Illumina sequencing | ITS1 | Genera: Mucor; Phoma; Cryptococcus; Tilletiopsis; Aspergillus; Penicillium; Acremonium; Aureobasidium | Shen et al. (2018a) |
| Apple | Fungi | Fruit | Mycotoxin contamination associated with fungal flora of apple core | AB3730xl sequencing | ITS1 and ITS4 | Genera: Alternaria; Penicillium; Aspergillus; Fusarium; Trichoderma; Epicoccum; Diplodia; Davidiella; Nigrospora; Paraconiothyrium; Bionectria; Botrytis | Soliman et al. (2015) |
| Arugula, Broccoli, Cabbage, Cauliflower, Horseradish; Radish and Turnip | Bacteria | Leaf | Microbiomes of vegetables related to plant disease resistance and human health | Illumina sequencing | V4 | Genera: Sphingopyxis; Sphingomonas; Pseudoxanthomonas; Pedobacter; Flavobacterium; Pseudomonas; Acinetobacter; Sphingomonas; Leuconostoc; Acinetobacter; Janthinobacterium; Achromobacter; Methylobacterium | Wassermann et al. (2017) |
| Asparagus | Bacteria | Stem | High‐hydrostatic pressure treatment for postharvest preservation | Pyrosequencing | V1–V3 | Genera: Streptococcus; Rubrobacter; Paucibacter; Janibacter; Escherichia; Clostridium; Citrobacter; Bacteroides; Propionibacterium; Brevibacterium; Sphingomonas; Pantoea; Bacillus; Tatumella; Rhodanobacter; Gammaproteobacteria; Halomonadaceae; Brenneria; Raoultella; Acetobacter; Pseudomonas; Proteobacteria; Serratia; Enterobacteriaceae; Rahnella; Acetobacteraceae; Leuconostoc; Gluconobacter | del Arbol et al. (2016a) |
| Baby Spinach | Bacteria | Leaf | Bacterial communities changed by the chlorine dioxide (ClO2) treatment | Illumina sequencing | V3‐V4 | Genera: Bacillus; Pseudomonas; Massilia; Pantoea | Truchado et al. (2018) |
| Bean and Radish | Bacteria | Seed | Bacteria community in the early plant developmental stages | Illumina sequencing | Not mentioned | Genera: Pantoea; Pseudomonas; Enterobacer; Ervinia; Pantoea; Streptomyces; Arthrobacter; Bacillus | Torres‐Cortes et al. (2018) |
| Cabbage | Bacteria | Leaf | Microbiota changes during cabbage ferment progress | Illumina sequencing | V3‐V4 | Genera: Leuconostoc; Lactobacillales; Bacillus; Chryseobacterium; Pseudomonas; Enterobacteriaceae; Enterobacteriaceae; Pseudomonas; Chryseobacterium; Pseudomonas | Einson et al. (2018) |
| Carrot | Bacteria | Root | Postharvest gamma irradiation affects surface carrot bacterial community | Illumina sequencing | V4‐V5 | Genera: Pseudomonas; Yersinia; Janthinobacterium; Bacillus; Escherichia; Geobacillus; Bacillales; Pseudomonadaceae; Enterobacteriaceae; Ureibacillus | Dharmarha et al. (2019b) |
| Cherry | Bacteria | Fruit | High‐hydrostatic pressure treatment affects bacterial community | Pyrosequencing | V1–V3 | Genera: Streptococcus; Rubrobacter; Paucibacter; Janibacter; Escherichia; Clostridium; Citrobacter; Bacteroides; Propionibacterium; Brevibacterium; Sphingomonas; Pantoea; Bacillus; Tatumella; Rhodanobacter; Gammaproteobacteria; Halomonadaceae; Brenneria; Raoultella; Acetobacter; Pseudomonas; Proteobacteria; Serratia; Enterobacteriaceae; Rahnella; Acetobacteraceae; Leuconostoc; Gluconobacter | del Arbol et al. (2016b) |
| Cherry | Bacteria | Fruit | High‐hydrostatic pressure processing changed the microbiological quality and bacterial biodiversity | Pyrosequencing | V1‐V3 | Genera: Enterobacteriaceae; Rahnella; Acetobacteraceae; Leuconostoc; Gluconobacter | Toledo del Arbol et al. (2016) |
| Clementines, Potatoes and Tomatoes | Bacteria | Fruit and Root | Food packaging materials affected microbial community | Illumina sequencing | V3‐V4 | Phylum: Proteobacteria; Firmicutes; Actinobacter; Bacteroidetes; Verrucomicrobia; Terenicutes; Plantomycetes | Brandwein et al. (2016) |
| Grape | Bacteria | Fruit | Bacterial community and its temporal succession during the fermentation | Pyrosequencing | V1–V3 | Genera: Gluconobacter; Pedobacter; Spingomonas; Janthinobacterium; Pseudomonas | Piao et al. (2015) |
| Grape | Bacteria | Fruit | Diversity and evolution of microorganisms during grape wine fermentation | Pyrosequencing | Not given | Genera: Orbus; Bifidobacterium; Wolbachia; Acetobacter; Gluconacetobacter; Lactobacillales; Gluconobacter | Portillo and Mas (2016) |
| Grape | Bacteria | Leaf and Stems | Human and animal pathogens related to foodborne diseases in the grapevine endosphere | Pyrosequencing | V5‐V9 | Genera: Propionibacterium; Staphylococcus; Clostridium; Burkholderia | Yousaf et al. (2014) |
| Grape | Fungi | Fruit | Changes in microbiota and mycobiota during spontaneous fermentation | Pyrosequencing | ITS1‐5.8S–ITS2 | Genera: Nakazawaea; Hanseniaspora; Zygosaccharomyces; Starmerella; Aureobasidium; Botryotinia; Pichia; Penicillium; Cladisporium; Thelebolus; Geuhomyces | Stefanini et al. (2016) |
| Grape | Fungi | Fruit | Microbial community in wine fermentation | Illumina sequencing | BITS and B58S3 | Genera: Alternaria; Aspergllus; Aureobasidium; Botryotinia; Brettanomyces; Candida; Cladosporium; Cryptococcus; Erysiphe; Hanseniaspora; Kluyveromyces; Lachancea; Metchnikowia; Meyerozyma; Mucor; Penicillium; Pichia; Rhodosporidium; Rhodotorula; Saccharomyces; Torulaspora; Wicherhamomyces; Zygosaccharomyces | Sternes et al. (2017) |
| Grape | Fungi | Fruit | Composition and changes of the fungal population during fermentation | Pyrosequencing | ITS1 | Genera: Sporobolomyces; Periconia; Peniophora; Leptosphaerulina; Issatchenkia; Bathalimia; Diaporthe; Botrytis; Pithomyces; Phoma; Leptospherulina; Cryptococcus; Alternaria; Zygosaccharomyces; Metschnikowia; Diplodia; Cytospora; Candida | Stefanini et al. (2017) |
| Grape | Fungi | Fruit | Wine fermentation affected by the metabolome | Pyrosequencing | ITS1 | Genera: Penicillium; Aspergillus; Botryosphaeria; Starmerella; Saccharomyces; Hanseniaspora; Cladosporium; Aureobasidium | Wang et al. (2015) |
| Grape | Fungi | Fruit | Soil microbial composition shifts based on under‐vine management, but no corresponding changes in fruits | Illumina sequencing | ITS1 | Genera: Penicillium; Sporobolomyces; Coprinellus; Ischnoderma; Mycosphaerella; Occultifur; Pestalotiopsis; Tilletiopsis | Chou et al. (2018) |
| Grape | Fungi | Fruit | Microbiome dynamics during spontaneous fermentations of sound grapes in comparison with sour rot and Botrytis infected grapes | PCR‐DGGE | 515F/806R | Genera: Aspergillus; Rhizopus; Saccharomyces cerevisiae; Starmerella; Penicillium; Zygosaccharomyces; Acetobacter; Ameyamaea; Gluconacetobacter; Gluconobacter; Tanticharoenia; Oenococcus; Botrytis; Cladosporium; Hanseniaspora | Lleixa et al. (2018) |
| Grape | Fungi | Fruit | Yeast communities on the grape berries affect by fungicides | PCR‐DGGE | ITS1 and ITS4 | Species: Aureobasidium Pullulans; Candida sp.; Candida Zemplinina; Cryptococcus Carnescens; Cryptococcus Dimennae; Cryptococcus Flavescens; Cryptococcus Magnus; Cryptococcus sp.; Cryptococcus Victoriae; Cryptococcus Wieringae; Hansenispora Uvarum; Issatchenkia Terricola; Metschnikowia Pulcherrima; Pichia Fermentans; Pichia Membrannifaciens; Rhodosporidium Babjevae; Rhodotorula Glutinis; Rhodotorula Nothofagi; Saccharomyces cerevisiae | Milanovic et al. (2013) |
| Grape | Fungi | Fruit | Diversity and evolution of microorganisms during grape wine fermentation | Pyrosequencing | Not given | Genera: Hanseniaspora; Saccharomyces; Issatchenkia; Candida; Saccharomycodes | Portillo and Mas (2016) |
| Lettuce | Bacteria | Leaf | Chitin amendment changed the microbial composition | Illumina sequencing | V3‐V4 | Genera: Cellvibrio; Sphingomonas; Pedobacter; Azospirillum; Taibaiella; Nitrospira; Streptomyces; Nocardioides | Debode et al. (2016) |
| Lettuce | Bacteria | Leaf | Gamma irradiation changed the phyllosphere microbiota | Illumina sequencing | V4‐V5 | Genera: Pseudomonas; Ureibacillus; Escherichia; Bacillus; Symbiobacterium; Thermoactinomycetes | Dharmarha et al. (2019a) |
| Lettuce | Bacteria | Leaf | Innovative washing solutions shifted the lettuce microbiota | Pyrosequencing | V1‐V3 | Genera: Flavobacterium; Pseudomonas; Pedobacter; Oxalabacteraceae; Comamonadaceae; Chryseobacterium; Agrobacterium; Sphingomonas; Janthinobacterium; Methylophilaveae | Siroli et al. (2017) |
| Lettuce | Fungi | Leaf | Chitin amendment changed the microbial composition | Illumina sequencing | ITS2 | Genera: Morteriella; Lecanicillium; Pseudogymnoascus; Pesudeurotium | Debode et al. (2016) |
| Maize | Fungi | Seed | Composition and variation in the fungal communities associated with seeds during storage | AB3730xl sequencing | ITS1/ITS4 | Genera: Aspergillus; Fusarium; Penicillium; Alternaria; Trichoderma; Chaetomium; Botryothichum; Cladosporium; Bipolaris; Rhizopus | Xing et al. (2018) |
| Orange Peel | Fungi | Fruit | Dynamic of fungi communities during the storage | Illumina sequencing | ITS1/ITS4 | Genera: Cyphellophora; Epicoccum; Meyerozyma; Saccharomycopsis; Trichomerium; Colletotrichum; Meira; Cryptostroma; Alternaria; Zymoseptoria; Bryochiton; Talaromyces; Acaromyces; Cosmospora; Mycosphaerella; Pseudopithomyces; Paraphaeosphaeria; Strelitziana; Cyclocybe; Pichia; Aspergillus; Cladosporium; Hanseniaspora; Ceramothyrium; Schwanniomyces; Cystofilobasidium; Penicillium; Candida; Microidium; Wallemia | Wang et al. (2018a) |
| Peanut | Fungi | Seed | Dynamic of fungi communities during the peanut storage | Illumina sequencing | ITS1 | Genera: Ascomycota; Talaromyces; Nectriaceae; Ceratobasidiaceae; Clonostachys; Enericella; Eurotium; Phizopus; Penicillium; Aspergillus | Ding et al. (2015) |
| Pear | Bacteria | Fruit | Chlorine drenching and after controlled atmosphere storage affect bacterial populations | AB3730xl sequencing | F‐27, R‐1492 | Genera: Curtobacterium, Pseudomonas, Pantoea; Erwinia Billingiae; Frigoribacterium | Duvenage et al. (2017) |
| Spinach | Bacteria | Leaf | Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage | Pyrosequencing | V4 | Genera: Deinococcus; Exiguobacterium; Sphingomonas; Methylobacterium; Rhizobium; Brevundimonas; Acinetobacter; Pseudomonas; Stenotrophomonas; Pantoea; Escherichia; Ralstonia; Naxibacter; Massilia | Lopez‐Velasco et al. (2011) |
DNA sequencing
First‐generation sequencing
The initial DNA sequencing methodology was developed in the mid‐1970s. Sanger et al. established a method for determining DNA sequences via primed synthesis with DNA polymerase (Sanger et al., 1974; Sanger and Coulson, 1975). Progress in Sanger’s method was made after the introduction of chain‐terminating inhibitor dideoxyribonucleotides (ddNTPs; Sanger et al., 1977). In the same year, Maxam and Gilbert reported a DNA sequencing approach based on chemical modification and specific cleavage (Maxam and Gilbert, 1977). The first‐generation DNA sequencing method of shotgun sequencing was developed based on the principles of both methods (Morey et al., 2013). This technique involves the following steps (Fig. 1). A DNA template is broken down into fragments. Each DNA fragment is then amplified (initially by E. coli cloning and later by PCR). The amplicons of the DNA fragments are then sequenced in a system with four independent PCR arrays. In the four independent PCR assays, four chain‐terminating inhibitors (ddATP, ddTTP, ddGTP and ddCTP) are added. In the PCR assays, the majority of DNA polymerase reactions are conducted by adding dNTPs. However, in several reactions, ddNTPs are added for polymerization, thus terminating chain extension. As a result, DNA amplicons with different lengths are obtained. An electrophoresis method is used to separate the terminated DNA amplicons. The continuous DNA sequence is obtained by docking the ends of the electrophoresis‐separated products.
Fig. 1.
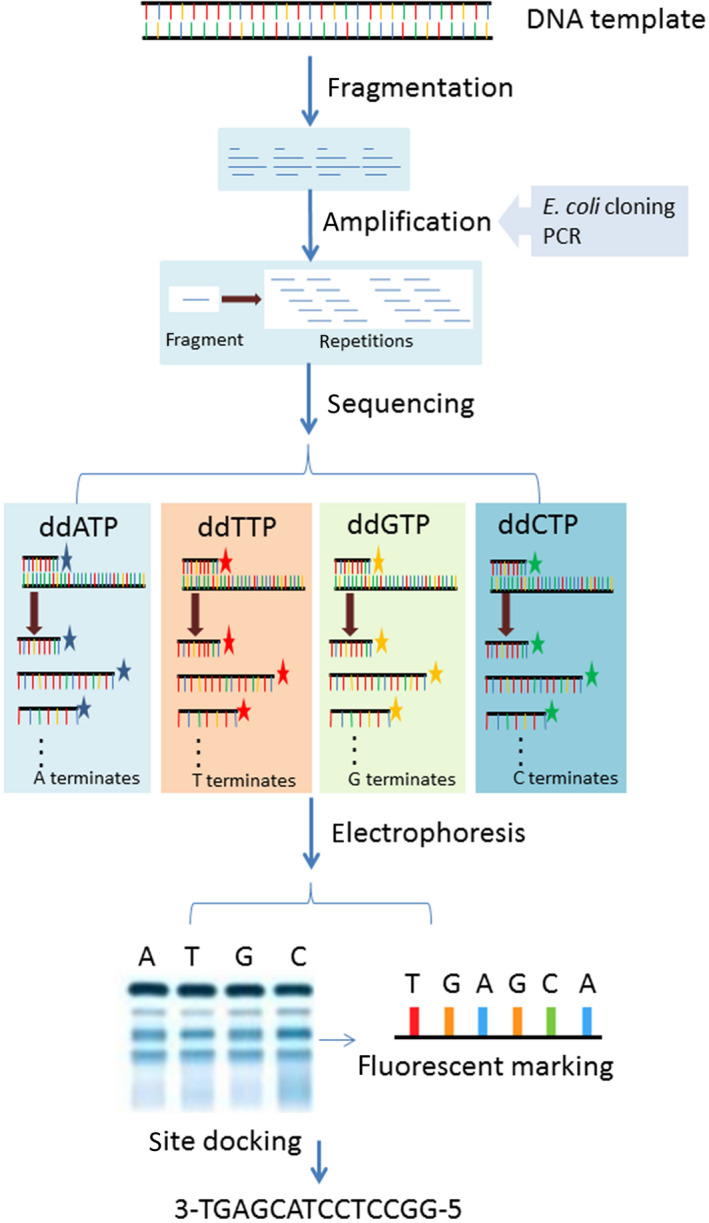
Schematic representation of first‐generation DNA sequencing.
Automated DNA sequencing has been available since the introduction of fluorescently labelled ddNTPs and spectrum detection in the mid‐1980s. The first automated DNA sequencer (AB370) was announced by Applied Biosystems Co. in 1987 (Bankier et al., 1991). The read length produced by the AB370 platform reached 600 bp, with detection of 96 bp per cycle and 500 kb per day. The AB370 system was upgraded to AB3730xl platform in 1995, with an improved read length (approximately 900 bases), speed (2.88 Mb per day) and accuracy (over 99.99%; Liu et al., 2012). However, compared with NGS platforms, the AB3730xl system was far from meeting the needs for a higher speed, throughput and cost‐effectiveness.
Next‐generation sequencing
Three NGS platforms were introduced during the first decade of the 21st century: the Roche 454 pyrosequencing, Illumina Genome Analyzer and SOLiD sequencing platforms. Compared with FGS, these NGS technologies perform multiparallel sequencing, which improves the throughput and speed and reduces costs.
Roche 454 pyrosequencing was announced by the 454 Life Sciences Co. in 2005. Pyrosequencing is based on sequencing by synthesis and relies on the detection of the released pyrophosphate when a nucleotide polymerizes to the nascent DNA chain (van Dijk et al., 2014). The template DNA is divided into 300‐ to 500‐base single‐stranded fragments (Fig. 2). Each DNA fragment was connected with two specific adapters at both ends and then attached to a 20 μm bead and transferred to a PTP hole (29 μm). The bead is emulsified to form a water‐in‐oil structure. The DNA fragment is amplified by performing emulsion PCR to form thousands of repetitions. In a single pyrosequencing cycle, four dNTPs (dATP, dGTP, dCTP and dTTP) are added to the PTP hole, and only one of them correctly matches the leading chain and is integrated into the nascent DNA. As soon as the correct dNTP is added and polymerized, the pyrophosphate is released to trigger luciferase‐mediated light emission. The signal is captured by a spectrum detector. The addition of the other three unpaired dNTPs does not generate a signal, and they will subsequently be removed. The combined data from hundreds of pyrosequencing cycles are used to generate DNA sequence reads. Thousands of PTP holes are arrayed on a PTP board. Therefore, numerous pyrosequencing reactions can be conducted simultaneously.
Fig. 2.
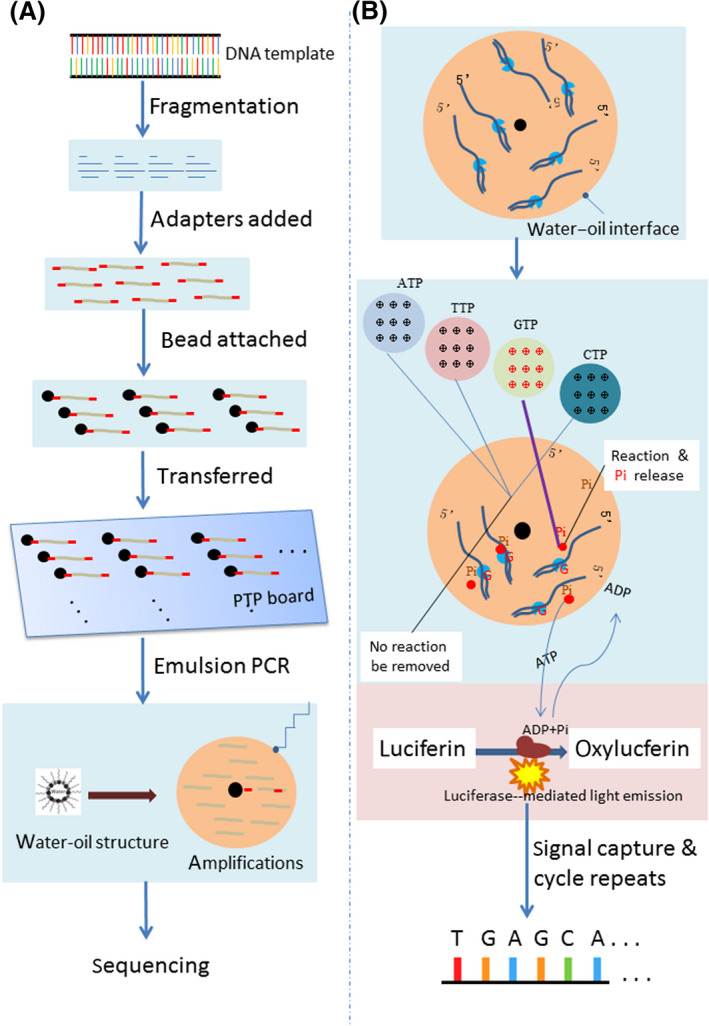
Schematic representation of Roche 454 pyrosequencing. Steps of DNA library preparation (A). Steps of pyrosequencing (B).
The Illumina Genome Analyzer was first introduced by Solexa in 2006 and purchased by Illumina in 2007. The Illumina platform is based on sequencing by synthesis (Morey et al., 2013; Ghanbari et al., 2015). The template DNA is first divided into 100‐ to 200‐base single‐stranded fragments. Both ends of the fragment are connected with an oligonucleotide adaptor. The two adaptors are complementarily matched with forward and reverse primers immobilized on a glass surface. The DNA fragment is amplified by performing bridge PCR to generate thousands of repeats. Repeats from a single DNA fragment form a separate strand by linearization. DNA sequencing is then performed by using four specific dNTPs. The dNTPs contain a specific cleavable fluorescent blocking group at the 3′‐OH end. In each sequencing cycle, the incorporation of a dNTP into the nascent DNA stimulates the release of a fluorescent signal, which is captured by a detector. Then, the blocking group on 3′‐OH is removed to continue the next sequencing step. Combined signals from hundreds of sequencing cycles are used to generate DNA sequence reads.
The SOLiD platform was introduced by Applied Biosystems in 2007. In contrast to sequencing by synthesis, SOLiD relies on the method of sequencing by ligation (Morey et al., 2013). The NDA library preparation method is similar to Roche 454 pyrosequencing. The template DNA is first broken down into 30‐ to 50‐base single‐stranded fragments. Each DNA fragment linked with two adaptors at both ends and then attached to a 1 μm bead. The bead is immobilized on a glass slide. The DNA fragment is amplified by performing emulsion PCR. Sequencing is performed by using sixteen classes of 8‐base fluorescent nucleotide probes and 5 classes of primers. In the 8‐base probe, the 1st and 2nd positions at the 3′‐end can be occupied by any combination A, T, G or C, resulting in a total of 16 probes. The 8th probe position is linked with four fluorescent groups, which correspond to the first two nucleotides at the 3′‐end (mainly the 1st position). The 3rd and 4th positions of the probe can match any base. The 5th nucleotide is the cleavage site. Five universal primers match the continuous positions (n, n + 1, n + 2, n + 3 and n + 4) on the template DNA. Sequencing is initiated by hybridization. The first primer is hybridized to the template DNA (the initial site, n). Then, an 8‐base probe is introduced, which correctly matches the 1st and 2nd positions of the template DNA. Therefore, the fluorescent signal from the probe reflects the nucleotides of the 1st and 2nd positions (mainly the 1st position). After recording the signal, the fluorescent group is removed by cutting at the 5th position of the probe. Another probe matching the 6th and 7th positions of template is connected at the 5th cleavage site. Therefore, the second fluorescent signal reflects the 6th and 7th nucleotides. These steps are repeated until the ligation reaction is complete. The signals of the original consecutive fluorescent codes (n) are obtained, followed by melting. The second universal primer (n + 1) is used to obtain the corresponding fluorescent codes of the 2nd and 3rd positions, the 7th and 8th positions and so on. Then, three other universal primers (n + 2, n + 3 and n + 4) are used to obtain the three consecutive corresponding fluorescent codes. By combining the five signals (n, n + 1, n + 2, n + 3 and n + 4) of the colour codes and the read matrix, the original sequence of the template DNA can be calculated.
The three NGS platforms exhibit different characteristics (Liu et al., 2012). Roche 454 sequencing produces a maximum read length of approximately 700 bases, which is longer than the read lengths generated by Illumina sequencing (150–200 bases) and SOLiD (30‐50 bases). In addition, Roche 454 sequencing is faster than Illumina or SOLiD sequencing. However, the Roche 454 platform presents an insufficient sequencing accuracy. Because dNTPs are not added to the last base of the DNA lagging chain, the last base of the sequence cannot be read. The Illumina sequencing platform exhibits the highest sequencing throughput and lowest operating cost per base. However, Illumina sequencing produces a shorter read length and therefore requires a greater sequencing depth. The two‐base coding and verification system of the SOLiD platform exhibits the greatest accuracy. However, the computing steps for the colour‐coding matrix and the combination of iterative data are complicated. Moreover, the shorter read length of this platform requires a greater sequencing depth. Several shortcomings commonly emerge in NGS platforms (Pushkarev et al., 2009). Generally, the shorter read length of NGS requires that the template DNA be highly fragmented. This demands a greater sequencing depth and complex data computing for obtaining the overall reads. Second, PCR amplification is strictly required. However, PCR results are often inconsistent in regions such as those with a higher GC% or repeated hairpins. PCR amplicons also show variations in abundance under uniform PCR procedures (van Dijk et al., 2018).
Third‐generation sequencing
There are three leading TGS technologies: the HeliScope Single Molecule Sequencer (SMS), the single‐molecule real‐time (SMRT) approach and the Oxford Nanopore MinION sequencer (Reuter et al., 2015; Lu et al., 2016; van Dijk et al., 2018). Compared with NGS, the fundamental improvement achieved by TGS is that a single strain or longer DNA molecules can be sequenced without PCR amplification. In addition, TGS technology shows real‐time performance, a higher throughput and reduced costs.
The HeliScope SMS was introduced by Helicos BioSciences in 2009 (Pushkarev et al., 2009; Reuter et al., 2015). The HeliScope SMS approach is based on sequencing by single‐molecule synthesis. The template DNA is first divided into single‐stranded fragments. Then, the 3′‐end fragment is linked to a poly‐A tail. Sequencing is performed on a HeliScope slide containing millions of flow cells. The flow cells contain a fixed with a poly T tail that both hybridizes with poly‐A sequence of the DNA template and provides a primer for DNA synthesis. Sequencing is initiated by the addition of the four fluorescently labelled and 3′‐OH‐blocked dNTPs, which is similar to Illumina sequencing. In each sequencing cycle, four dNTPs are added in turn. Once the correct dNTP is added and polymerized, the fluorescent signal is released. The signal is captured by a highly sensitive detection system. Then, the blocking group on the 3′‐OH is removed to begin the next sequencing step.
The SMRT TGS platform was launched by Pacific Bioscience in 2011 (van Dijk et al., 2018). SMRT sequencing is based on DNA synthesis, and the signal is captured via zero‐mode waveguide (ZMW) detection (Fig. 3). The ZMW nanopore is a channel with a diameter of 10 nm, which provides limited space for DNA polymerization. At the bottom of the ZMW nanopore, a DNA polymerase is immobilized. The template DNA is disintegrated into single‐stranded fragments of tens of kb. Both ends of a fragment are connected to two closed circular single‐stranded DNA adaptors. The DNA fragment is then introduced into the nanopore and ligated to the polymerase via either adaptor. Four fluorescently labelled and 3′‐OH‐blocked nucleotides are added to the reaction cells to start the synthesis process. Immediately after nucleotide polymerization, a fluorescent signal is generated. At the same time, laser irradiation of the nanopore is performed, and the fluorescent signal is amplified to a detectable level and transmitted to the nanopore‐external space. Thus, the undetectable fluorescent signal in the ZMW pore can be captured. Once the blocking group on 3′‐OH is removed, the next sequencing cycle continues. There are approximately 150 000 ZNWs in an SMRT unit, which is enough to obtain a sufficient throughput.
Fig. 3.
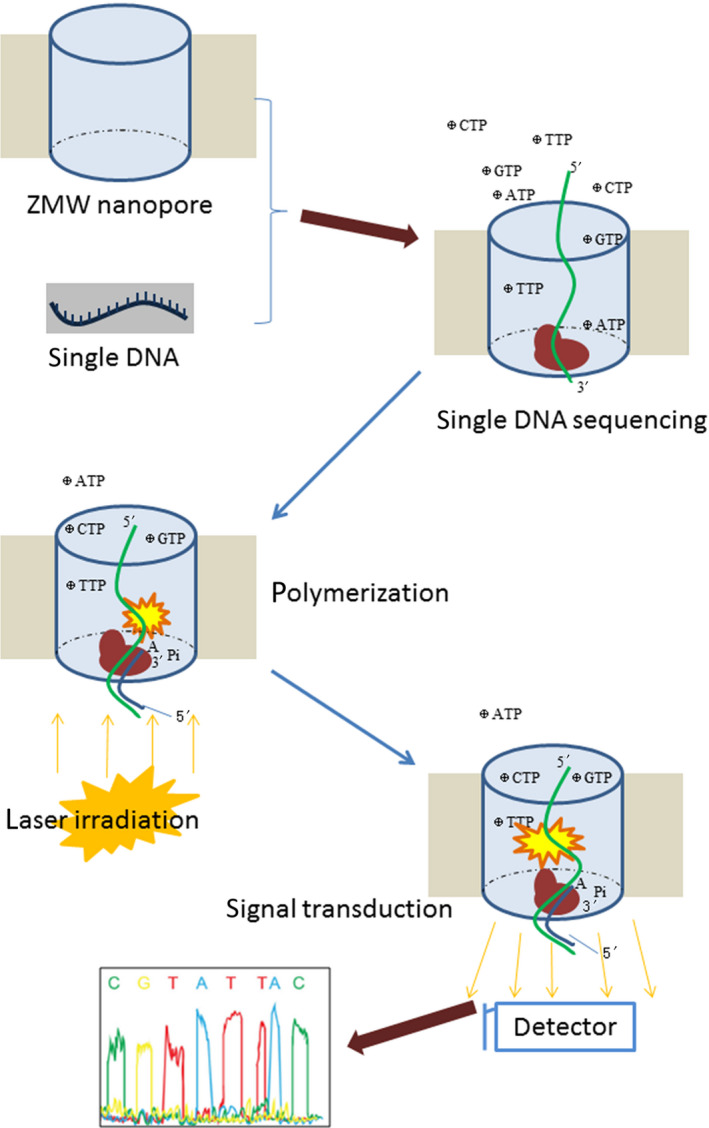
Schematic representation of SMRT sequencing.
MinION sequencing was introduced by Oxford Nanopore Technologies in 2014 (Mikheyev and Tin, 2014). MinION sequencing is based on DNA electrophoresis, in which a‐haemolysin nanopores distributed across a semipermeable membrane serve as channels for DNA electrophoresis. Cyclodextrins covalently bind to the nanopores to increase nucleotide‐channel interactions. First, the template DNA is fragmented by using Covaris g‐TUBEs to form single‐stranded DNA fragments. The two ends of the DNA fragment are connected with two adapters. The lead adapter (Y adapter) is added to the 5′‐end, and the hairpin adapter (HP adapter) is added to the 3′‐end. An electric field is applied to both sides of the membrane to provide a driving force for DNA cross‐channel electrophoresis. Driven by voltage, DNA fragments enter and pass through the pores and interact with cyclodextrin in the process. Different nucleotide bases (A, T, G and C) interact with cyclodextrin differently and generate corresponding current waves. The ion current is measured and characterized to obtain the sequence of template DNA.
The three TGS platforms have different characteristics. The HeliScope SMS has not been widely used, because of its relatively slower speed, shorter read length and higher price. SMRT is the most commonly used TGS approach. In recent years, SMRT technology has been highly improved to achieve a sufficient throughput and cost‐effectiveness. The SMRT PacBio RSII platform produces a read length of 10–15 kb and a throughput of 0.5–1.0 Gb per run (van Dijk et al., 2018). The read length of Nanopore MinION sequencing is similar to that of PacBio RSII. However, the error rate of Nanopore MinION (20–40%) is higher than that of PacBio RSII (10–15%; Lu et al., 2016). However, the Nanopore MinION sequencer has attracted considerable interest due to its smaller size, cheaper equipment and lower running costs.
WGS and genetic marker identification of related microbes on F & V
WGS and genetic marker identification of related foodborne pathogen
The Escherichia genus contains three species: E. coli, E. albertii and E. fergusonii. E. coli exhibits many strains, which usually colonized the intestines of humans and other mammals and are considered to be part of the intestinal flora. Gut E. coli can be released into the environment via feces. Therefore, the count of E. coli can reflect the extent of fecal contamination. In addition, several E. coli strains are serious opportunistic pathogens that cause food poisoning. To date, 17 952 E. coli genomes have been registered (Fig. 4), and approximately 1000 representative references have been summarized (NCBI genome ID 167). The first E. coli genome was obtained for strain K‐12 MG1655 by shotgun sequencing (Blattner et al., 1997). Subsequently, Hayashi et al. reported the genome of the pathogenic strain E. coli O157:H7 RIMD 0509952 (Hayashi et al., 2001). The genome of E. coli O157:H7 is 859 kb larger than that of strain K‐12 MG1655, and the comparison of their genomes showed extensive polymorphisms (Hayashi et al., 2001). There are fewer reports of E. albertii and E. fergusonii as pathogens responsible for food poisoning. Genome sequencing has revealed 89 and 18 strains of E. albertii and E. fergusonii respectively (NCBI data). The representative E. albertii strain KF1 was the first to be sequenced and reported (NCBI genome ID 1729; Fiedoruk et al., 2014). 16S rDNA sequencing has been used to distinguish E. coli, E. albertii and E. fergusonii (Maifreni et al., 2013). Multiplex PCR based on the cdgR, EAKF1_ch4033 and EFER_0790 genes can efficiently distinguish E. coli, E. albertii and E. fergusonii (Lindsey et al., 2017). For the identification of E. coli, the reported genetic markers mainly include fliC, Vt1, Vt2 (Gannon et al., 1997), uspA (Osek, 2001), lacZ (Foulds et al., 2002), rfbE, eae, stx1, stx2 (Ooka et al., 2009), ipaH (van den Beld and Reubsaet, 2012), lacY, uidA (Mendes Silva and Domingues, 2015), PhoA (Yang et al., 2016) and cdtB (Hassan et al., 2018; Table 1). The stx1, stx2 and eae genes have been reported as specific virulence markers for enterohemorrhagic E. coli O157 (Franz et al., 2007; Ooka et al., 2009). The verotoxin genes (VT1 and VT2) serve as markers for specific VT‐producing E. coli (Gannon et al., 1997). E. albertii can be identified based on the specific 16S rDNA (Grillova et al., 2018), cytolethal distending toxin (cdtB) gene (Maheux et al., 2014) and cysteine biosynthesis gene (EAKF1_ch4033; Lindsey et al., 2017) sequences. The regions of yliE, EFER_1569 and EFER_3126 are efficient for the multi‐PCR detection of E. fergusonii (Simmons et al., 2014).
Fig. 4.
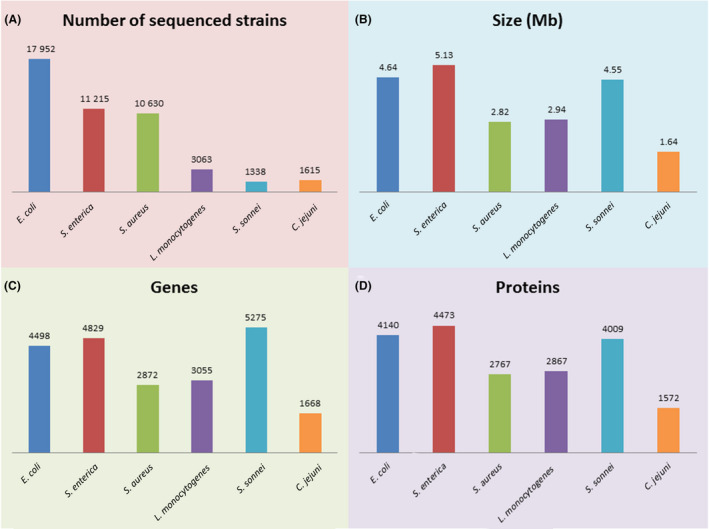
Genomic information for major foodborne pathogen species of Escherichia coli (E. coli), Salmonella enterica (S. enterica), Staphylococcus aureus (S. aureus), Listeria monocytogenes (L. monocytogenes), Shigella sonnei (S. sonnei) and Campylobacter jejuni (C. jejuni).
The Salmonella genus contains two pathogenic species, and S. enterica and S. bongori. S. enterica can be subdivided into six subspecies: enterica (I), salamae (II), arizonae (IIIa), diarizonae (IIIb), houtenae (IV) and indica (VI; Agbaje et al., 2011; Lamas et al., 2018). Alternatively, S. enterica can be subdivided into approximately 2,500 different serotypes according to somatic (O) and flagellar (H) antigens (Tindall et al., 2005; Grimont and Weill, 2007). S. enterica subspecies I causes the majority of cases of foodborne diarrhoea (Sanchez‐Vargas et al., 2011; Rezende et al., 2016). S. enterica subspecies I exhibits approximately 1531 serotypes (Desai et al., 2013; Lamas et al., 2018). To date, approximately 11 215 strains of S. enterica have been registered (Fig. 4), and approximately 340 representative references concerning S. enterica genomic research have been summarized (NCBI genome ID 152). The genome of the representative S. enterica strain Typhi str. CT18 was the first to be reported (Parkhill et al., 2001). Detailed genomic comparisons between S. enterica typhi, E. coli and S. typhimurium have been reported (McClelland et al., 2001). The genomes of approximately 20 strains of S. bongori have been registered, among which the representative strain is S. bongori NCTC 12419 (NCBI genome ID 1089). The sseL, spvC (Peterson et al., 2010), avrA, stn, stm (Amin et al., 2016), spv, hut, fljB (Alzwghaibi et al., 2018), hilA, fimA and hns (Jeyasekaran et al., 2011) gene regions have been reported as markers for the Salmonella genus. In addition, multiplex PCR analysis based on the fljB, gatD, invA, mdcA, stn and STM4057 genes is used for Salmonella genus identification (Lee et al., 2009). For S. enterica species, the AceK, fliC, invA, oriC, sdf, sefA, ssaN, STM2745, STM4492 and 16S rRNA genes are widely used for PCR identification and detection (Lee et al., 2009; Postollec et al., 2011). The fliC, gnd, and mutS genes are used to distinguish S. bongori from other Salmonella pathogens (Soler‐Garcia et al., 2014).
The Staphylococcus genus contains several species related to skin infection and food poisoning (mainly S. aureus, S. epidermidis, S. lugdunensis, S. saprophyticus and S. pseudintermedius). Specifically, S. aureus is an important pathogen associated with toxin‐related food poisoning. As of recently, the genomes of approximately 10 630 S. aureus strains have been registered (Fig. 4), and approximately 300 representative references have been summarized (NCBI genome ID 154). The genomes of two S. aureus strains, N315 and Mu50, were the first to be determined by performing shotgun sequencing (Kuroda et al., 2001). The characteristics of the genomes of potentially pathogenic species including S. epidermidis, S. lugdunensis, S. saprophyticus and S. pseudintermedius have been summarized (Table 1). Specific regions of mecA, nuc, femA‐SA, femA‐SE, orfx‐SCCmec, spa, gyrB and 16S rRNA are used as markers for S. aureus identification (Hirvonen, 2014). Based on polymorphisms in the femA gene, S. aureus and S. epidermidis can be differentiated (Jukes et al., 2010). Recently, multilocus sequence typing (MLST) was performed on femA, tuf, rpoB, gap, pyrH and ftsZ to identify Staphylococcus strains accurately (Song et al., 2019). The staphylococcal enterotoxin (se) genes of sea, seb, sec and see have been used to monitor toxic S. aureus in food (Omwenga et al., 2019).
The Listeria genus contains the pathogenic species L. monocytogenes and L. seeligeri. L. monocytogenes is an important foodborne pathogen that contaminates various F & V and causes human listeriosis (Buchanan et al., 2017). About the genomes of 3063 strains of L. monocytogenes have been registered to date (Fig. 4), and nearly 80 genomic references have been summarized (NCBI genome ID 159). The genome of the representative L. monocytogenes strain EGD‐e was the first to be obtained (Glaser et al., 2001). L. seeligeri is reported less often in food, and its genomic information is summarized in Table 1. Methods for L. monocytogenes identification have been reviewed previously (Gasanov et al., 2005; Välimaa et al., 2015). PCR‐based detection has mainly been performed for the hly, iap, mpl, prfA, inlA, inlB, actA (Gasanov et al., 2005), plcA and 16S RNA genes (Xu et al., 2008).
The Shigella genus contains four foodborne pathogens, S. flexneri, S. boydii, S. sonnei and S. dysenteriae (Warren et al., 2006). Shigella pathogens contaminate a variety of foods and exhibit diverse occurrences and different epidemiologies (Warren et al., 2006; Levin, 2009; Lin et al., 2010). S. dysenteriae serotype 1 causes deadly epidemics, S. flexneri causes endemic infection, foodborne diseases associated with S. boydii occur mainly in developing countries, and foodborne diseases associated with S. sonnei occur in developed countries (Hale, 1991). To date, approximately 480 strains of S. flexneri (NCBI genome ID 182), 113 strains of S. boydii (genome ID 496), 1338 strains of S. sonnei (genome ID 417; Fig. 4) and 67 strains of S. dysenteriae (genome ID 415) have been registered (Table 1). The genome of S. flexneri strain 2a str. 301 was the first to be obtained (Jin et al., 2002). S. flexneri serotype 2a and E. coli K12 MG1655 share a high degree of genomic similarity (Stephens and Murray, 2001; Yang et al., 2005). They exhibit a common sequence of approximately 3 Mb (65% in E. coli) that encodes 2790 proteins. In PCR‐based detection assays, specific gene regions of ipaH, virA, ial and 16S rRNA have been used as targets for Shigella genus identification (Warren et al., 2006). The ipaH gene, encoding the invasive plasmid antigen H, is carried by four Shigella species (Dutta et al., 2001; Warren et al., 2006). Specific regions of the rfc gene of S. flexneri, the wbgZ gene of S. sonnei and the rfpB gene of S. dysenteriae have been used to differentiate the three Shigella species (Ojha et al., 2013). SSR markers that can distinguish Shigella species have also been identified (Sahl et al., 2015). A recent study differentiated all four Shigella species by performing multiplex PCR analysis of differentiated genes (Kim et al., 2017a), for which a putative restriction endonuclease gene specific to S. sonnei, a hypothetical protein gene specific to S. boydii and S. dysenteriae and a repressor protein gene specific to S. flexneri were used.
The Campylobacter genus includes two major species of foodborne pathogens, C. jejuni and C. coli. To date, about the genomes of 1615 strains of C. jejuni have been registered (Fig. 4), and nearly 100 representative references describing genomic research have been summarized (NCBI genome ID 149). The genome of C. jejuni is relatively small, with a low GC% (Table 1). The genome of the representative C. jejuni strain NCTC 11168 has been reported (Parkhill et al., 2000), and has been indicated to harbour only a few repeat sequences and no transposons, phage remnants or insertion elements (Parkhill et al., 2000; Dorrell et al., 2001). C. coli is another pathogen that shows a distinctive epidemiology (Gillespie et al., 2002). To date, 928 genomic sequences have been registered for C. coli, and nearly 40 representative references have been summarized (NCBI genome ID 1145). The hip, 16S rRNA, rrs, cdaF, porA, Hyp, cjaA, ceuE, hipO, mapA, ceuA, askD, glyA, lpxA, ccoN, ORF‐C sequence, rpoB, oxidoreductase gene, cdtA and pepT genes are widely used for the PCR identification of C. jejuni (Frasao et al., 2017). The other related genetic regions used for C. coli identification are summarized in Table 1.
WGS and genetic marker identification of related phytopathogenic fungi
The Penicillium genus contains several pathogenic species, particularly P. expansum, P. digitatum, P. griseofulvum, P. italicum and P. citrinum. The majority of these species are related to the postharvest decay of F & V. P. chrysogenum was the first sequenced species in this genus, as it is used as an industrial penicillin producer (van den Berg et al., 2008). P. expansum is an important pathogen that accelerates corruption in various produce species (Nie, 2017; Shen et al., 2018a). P. expansum also produces the mycotoxins patulin and citrinin. P. expansum strain R19 was the first to be sequenced (Yu et al., 2014). To date, the genomes of nine strains of P. expansum have been registered (NCBI genome ID 11336; Fig. 5). A representative genome was reported for P. expansum from strain MD‐8 (Ballester et al., 2015). P. digitatum causes postharvest green mould in citrus fruits (Marcet‐Houben et al., 2012). The genomes of two P. digitatum strains, PHI26 and Pd1, have been reported (Marcet‐Houben et al., 2012). P. italicum causes postharvest blue mould on citrus fruit (Deng et al., 2018). The representative strain of P. italicum PHI1 was reported (Ballester et al., 2015). Genomic comparison showed that P. expansum and P. italicum present differences in gene clusters related to secondary metabolism (Ballester et al., 2015; Li et al., 2015). Fifteen genes for patulin biosynthesis have been identified in P. expansum, which are located in a gene cluster (Ballester et al., 2015; Li et al., 2015). These genes and functions have been reviewed previously (Puel et al., 2010). Several methods, including RAPD (Schena et al., 2000), SNP (Piombo et al., 2018) and microsatellite analysis (Mohmed et al., 2010), have been used for distinguishing Penicillium species. The isoepoxydon dehydrogenase (IDH) gene is considered to be a useful marker for distinguishing patulin‐producing and nonproducing Penicillium species (De et al., 2016; Rharmitt et al., 2016). Several gene regions, such as the patF (Tannous et al., 2015), ITS (Hammami et al., 2017), Pepg1 (Ostry et al., 2018) and polygalacturonase genes (Hesham et al., 2011), have been used for the identification of P. expansum. The specific genes employed for the identification of P. digitatum, P. griseofulvum, P. italicum and P. citrinum are summarized in Table 2.
Fig. 5.
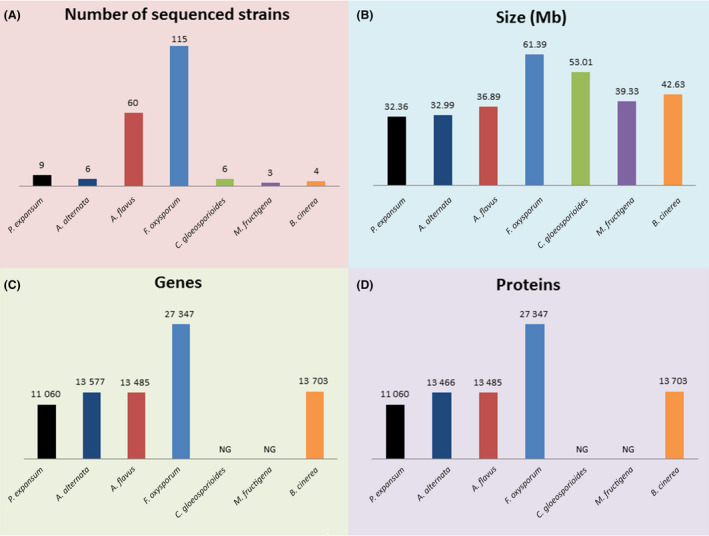
Genomic information for major phytopathogenic fungal species of Penicillium expansum (P. expansum), Alternaria alternata (A. alternate), Aspergillus flavus (A. flavus), Fusarium oxysporum (F. oxysporum), Colletotrichum gloeosporioides (C. gloeosporioides), Monilinia fructicola (M. fructicola) and Botrytis cinerea (B. cinerea).
The Alternaria genus contains several pathogenic species, particularly A. alternata, A. arborescens, A. brassicicola and A. solani. These pathogens commonly cause plant diseases and postharvest rot (Harteveld et al., 2014). Additionally, Alternaria species can produce host‐specific phytotoxins (HSTs), which differ between plant species (Akamatsu et al., 1999). Alternaria toxins are a group of mycotoxins produced by Alternaria species that mainly include tenuazonic acid (TeA), alternariol (AOH), alternariol monomethyl ether (AME), tentoxin (Ten) and altenuene (ALT). However, the genes responsible for Alternaria toxin biosynthesis have not yet been confirmed. To date, six strains of A. alternata have been registered (NCBI genome ID 11201; Fig. 5). Nguyen et al. (2016) reported the first draft genome of A. alternata ATCC 34957, obtained using PacBio SMRT technology, and discussed the gene regions related to mycotoxin metabolism. A. arborescens is another pathogen in this genus. Hu, et al. (2012) generated the first sequence for A. arborescens and demonstrated the horizontal transfer of the conditionally dispensable chromosome (CDC) carrying HST genes. The genome characteristics of A. arborescens, A. brassicicola, A. solani and A. tenuissima are summarized in Table 2. Several methods have been used for the identification of Alternaria species, such as high‐resolution melting (HRM) analyses, AFLP and SSR (Lorenzini and Zapparoli, 2014; Wolters et al., 2018). Genes such as histone‐3, glyceraldehyde 3‐phosphate dehydrogenase (Gpd), Alt a1, AaSdhB, AaSdhC, AaSdhD, ITS and β‐tubulin have been used for Alternaria species identification (Table 2). The Alt a1 gene is a widely used marker for Alternaria species (Gabriel et al., 2017). The polyketide synthetase (PKS) gene and nonribosomal peptide synthesis (NRPS) gene are essential for Alternaria toxin synthesis and regulation and can also be used to identify Alternaria toxin‐producing species (Chen et al., 2015).
The Aspergillus genus is large and contains several saprophytic/pathogenic species, particularly A. flavus, A. parasiticus, A. carbonarius, A. niger, A. tubingensis and A. westerdijkiae. These species cause corruption in various agricultural products and produce the aflatoxin and ochratoxin A mycotoxins. To date, the genomes of a total of 60 A. flavus strains have been registered (NCBI data, genome ID 360; Fig. 5). A representative genome of A. flavus was reported from strain NRRL3357 (Nierman et al., 2015). Genomic comparison between A. flavus strains NRRL3357 and AF70 showed polymorphisms in their aflatoxin toxin gene cluster (Sharma et al., 2018). A. parasiticus is another important aflatoxin producer. Two strains of A. parasiticus have been sequenced, and the representative strain is SU‐1 (NCBI genome ID 12976). Genomic comparison revealed approximately 98% similarity between the six A. flavus species and 81% similarity between A. flavus and A. parasiticus species (Faustinelli et al., 2016). Fourteen strains of A. niger have been registered, the majority of which have been related to citrate production or sugar metabolism (Aguilar‐Pontes et al., 2018; Laothanachareon et al., 2018). A. carbonarius is another ochratoxin A‐producing member of this genus, for which one strain has been sequenced (NCBI genome ID 947). As illustrated in previous reviews, the genes responsible for aflatoxin biosynthesis are integrated as a cluster that contains approximately 25 genes with a total length of 80 kb (Yu et al., 2004; Moore et al., 2010). This gene cluster contains the main regulatory genes aflR and aflS and the biosynthesis genes aflD, aflM, aflP, aflQ aflD, aflO and aflQ. Aflatoxin biosynthesis and regulatory genes have been widely used to identify toxin‐producing species (Mahmoud, 2015; Hua et al., 2018). Polymorphisms of the calmodulin gene have been used to identify Aspergillus species (Palumbo and O'Keeffe, 2015. The β‐tubulin gene can also be used for the specific identification of several Aspergillus species (Nasri et al., 2015; Falahati et al., 2016). The other genetic markers for Aspergillus species are summarized in Table 2.
The Fusarium genus contains several pathogenic species, particularly F. oxysporum, F. fujikuroi, F. verticillioides, F. proliferatum, F. graminearum and F. sporotrichioides. These pathogens cause serious diseases in crops and vegetables and produce toxic trichothecene mycotoxins. The genomes of a total of 115 F. oxysporum strains have been registered (NCBI genome ID 707; Fig. 5). The representative strain of F. oxysporum f. sp. lycopersici 4287 has been reported (Ma et al., 2010). Genome comparison between F. graminearum, F. verticillioides and F. oxysporum revealed genomic lineage‐specific (LS) regions in the Fusarium genus (Ma et al., 2010). F. fujikuroi is a plant pathogen that causes bakanae disease in rice and produces gibberellins (GAs). A total of 15 strains of this pathogen have been sequenced (NCBI genome ID 13188). The representative F. fujikuroi strain IMI 58289 has been reported (Wiemann et al., 2013). F. proliferatum is also a plant pathogen, and the genomes of 13 strains of this species have been registered (NCBI genome ID 2434). The representative strain F. proliferatum ET1 has been reported (Niehaus et al., 2016). For genetic detection, the Fusarium‐specific gene regions that have been used have mainly included the translation elongation factor‐1α (tef‐1α) gene (Wu et al., 2016), ITS (Jedidi et al., 2018), SIX (Debbi et al., 2018) and FUM gene (Omori et al., 2018) sequences. The genetic markers used for F. graminearum and F. sporotrichioides are summarized in Table 2.
The Colletotrichum genus contains several pathogenic species, particularly C. gloeosporioides, C. acutatum, C. coccodes and C. fructicola. C. gloeosporioides infects many crops, causing anthracnose diseases. A total of six strains of C. gloeosporioides have been registered, among which the representative strain is C. gloeosporioides SMCG1#C (NCBI genome ID 17739; Fig. 5). C. acutatum widely infects F & V (Cano et al., 2004; Heilmann et al., 2006). Two strains of this species have been registered (NCBI genome ID 38530). In addition, the genomes of two strains of C. coccodes and three strains of C. fructicola have been registered (Table 2). However, they were only registered at the scaffold level, and no related references have been reported. For PCR detection, the ITS, β‐tubulin, actin, act, ApMat, cal, chs1, gapdh, gs, his3, tub2, glyceraldehyde‐3‐phosphate dehydrogenase and coxidase subunit 1 gene have been widely used (Tapia‐Tussell et al., 2008; Chung et al., 2010; Ramdeen and Rampersad, 2013; Yang et al., 2015; Sharma et al., 2017).
The Monilinia genus contains several species related to brown rot on F & V, particularly M. laxa, M. fructicola, M. fructigena and M. polystroma. However, the genomes of Monilinia species have rarely been reported. To date, 2 strains of M. laxa, 2 strains of M. fructicola, 3 strains of M. fructigena (Fig. 5) and 1 strain of M. polystroma have been registered. The representative M. laxa strain 8L has been reported (NCBI data, genome ID 66927; Naranjo‐Ortiz et al., 2018). The inter‐simple sequence repeat (ISSR), RAPD (Fazekas et al., 2014) and HRM (Papavasileiou et al., 2016) methods have been used for interspecies identification. Specific regions of the cytochrome b gene (Ortega et al., 2019), laccase‐2 gene (Wang et al., 2018b), β‐tubulin gene (Fan et al., 2014), ITS (Guinet et al., 2016), MO368‐1, Laxa (Cote et al., 2004) and small subunit (SSU) rDNA (18S) gene (Fulton and Brown, 1997) have been reported as markers for PCR‐based detection.
The Botrytis genus contains the pathogenic species B. cinerea. B. cinerea causes serious grey mould diseases on F & V (Reich et al., 2016). Four strains of B. cinerea have been registered, among which the representative strain is B05.10 (NCBI genome ID 494; Fig. 5). Genomic comparative analysis between B. cinerea and Sclerotinia sclerotiorum revealed extensive genetic polymorphisms, but showed few significant polymorphisms in specific pathogenic clusters (Amselem et al., 2011). The RAPD and HRM methods have been used to identify B. cinerea (Thompson and Latorre, 1999). The genetic markers used for B. cinerea include the ITS (Reich et al., 2016), the necrosis and ethylene‐inducing protein gene (Munoz et al., 2016), the Bc‐hch locus (Zhang et al., 2018), G3PDH, HSP60 and RPB2 (Zhou et al., 2014), the necrosis and ethylene‐inducing protein 1 gene (Fan et al., 2015), the species‐specific sequence‐characterized amplified region (SCAR) marker (Suarez et al., 2005) and the intergenic spacer (IGS) region (Diguta et al., 2010).
16S rDNA and ITS sequencing of the microbiome community on F & V
16S rDNA and ITS sequencing‐based metagenomics
Metagenomic strategies are technological approaches that are increasingly being used to study the overall microbial community in complex biological samples (Cao et al., 2017). Significantly, metagenomics expands the scope of microbiology research and provides new insights into uncultivable microbes. This method is mainly based on polymorphisms in the bacterial 16S rDNA and fungal ITS regions, combined with powerful sequencing technologies, databases and software platforms (Fig. 6A). Total microbial DNA is directly extracted from research samples under this approach. rDNA PCR‐based denaturing gradient gel electrophoresis (PCR‐DGGE) was the first method developed to identify differential abundant microbes at a general level (Wang et al., 2015). The distinguished rDNA fragments are obtained by gel cutting, followed by sequencing. Then, the microbes are identified by performing sequence alignment against databases. In the 2000s, NGS could be used to sequence all of the obtained rDNA amplifications, which allows the microbial community to be analysed more deeply and comprehensively. The obtained sequences are assigned to operational taxonomic units (OTUs) based on similarity (Caporaso et al., 2010). Representative OTU sequences are identified (Fig. 6B). Additionally, OTU abundance provides relatively quantitative information for specific microbial taxa. Several analytical software platforms, such as the FLASH and QIIME packages, are used for downstream data analysis (Jünemann et al., 2017). To address the short read length and PCR dependence of NGS, TGS‐based metagenomics is promising and powerful (Uyaguari‐Diaz et al., 2016). The long reads obtained via TGS can be used to directly identify microbes at the species or even strain level. Additionally, the sequence abundance accurately represents the number of specific microbes. Once the cost is reduced, TGS will become a powerful tool for metagenomic research.
Fig. 6.
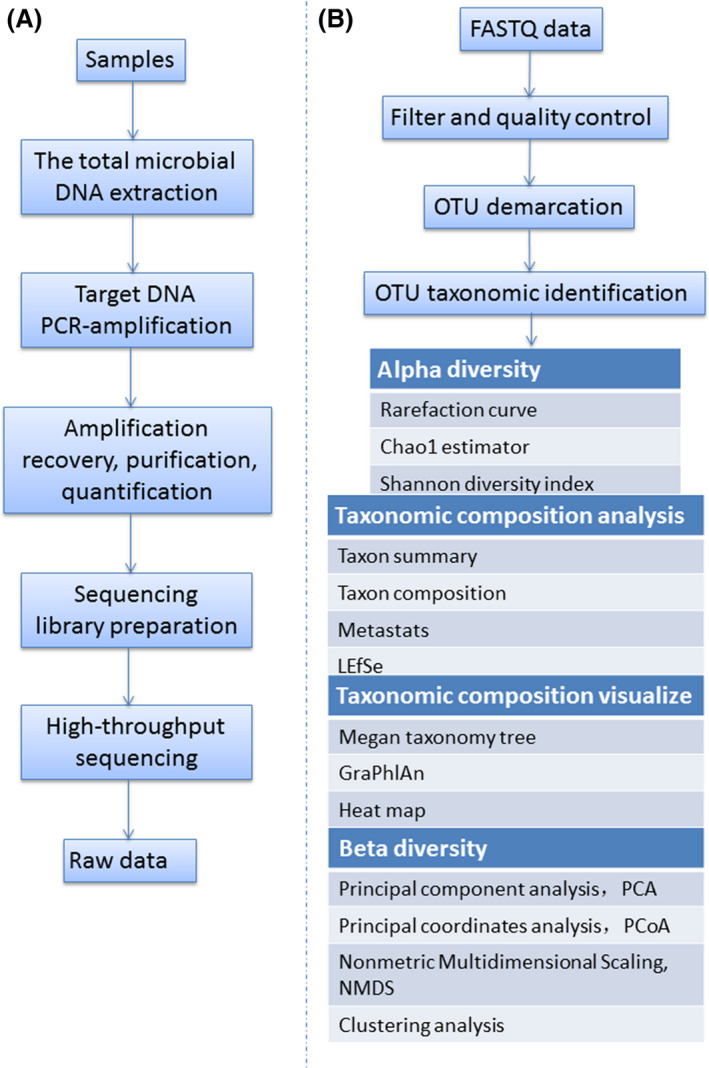
Basis of metagenomic analyses. General workflow for NGS‐based metagenomics (A). Bioinformatic analysis workflow for metagenomic data analysis (B).
Many studies have elucidated the bacterial and fungal communities on F & V by using 16S rDNA and ITS sequencing. For this review, we searched references mainly from the Web of Science, NCBI, ScienceDirect and CNKI platforms. A total of 64 original studies describing the microbiomes of various F & V were identified (Table 3). Illumina sequencing and Roche 454 pyrosequencing have been most widely used for these studies. PCR‐DGGE combined with AB3730xl sequencing was mainly used in earlier studies. Until recently, no TGS‐based metagenomic analyses of F & V had been reported. Based on common themes, we grouped these references into three categories: microbiome diversity between plant species/genotypes; regional/environmental factors and farming practices affecting microbiomes; and microbiomes affected by artificial treatment and quality control procedures in storage and processing.
Microbiome diversity between plant species/genotypes
Plant microbiomes are related to species/genotype specificity (Fig. 7). Recently, differential bacterial and fungal communities have been recorded on diverse fruits, including apples (Soliman et al., 2015), blueberries (Jiang et al., 2017), grapes (Pinto et al., 2014; Zhang et al., 2017a), kiwifruits (Purahong et al., 2018), spinach (Darlison et al., 2019) and tomatoes (Ottesen et al., 2013). Because of the importance of leafy vegetables in food control, the phyllosphere microbiomes have been monitored on vegetables such as spinach (Chen et al., 2018), lettuce (Higgins et al., 2018) and rocket (Darlison et al., 2019). In addition, the microbial community may be related to the specificity of plant tissue. The microbiomes differ among plant organs such as the fruits, leaves, flowers (Shade et al., 2013) and roots (Ottesen et al., 2013; Zhang et al., 2017b). However, they also show correlations between several plant tissues (Zhang et al., 2017b). The microbial community on F & V products is related to different characteristics and biological processes. In particular, the bacterial communities on several wine grape varieties have been found to differ, which may affect the fermentation process in winemaking (Bokulich et al., 2014). Additionally, the microbial communities of plants might be related to their chemical compositions. Recently, relationships between phyllosphere minerals and microbial communities have been observed in spinach and rocket (Darlison et al., 2019). Bacterial communities present in the plant rhizosphere could potentially be used as indicators of the soil environment and mineral efficiency (Jiang et al., 2017). Microbiome–host or microbiome–mineral interactions might be widespread. These NGS‐based metagenomic studies have shown that the microbiomes of F & V are plant specific.
Fig. 7.
Microbiome specificity in various plant species and tissues.
Microbiomes affected by regional/environmental factors and farming practices
The microbiomes of F & V are affected by differences in regional environments, farming practices and disease occurrences (Fig. 8A). Many environmental factors influence microbial activities, including temperature, humidity, light exposure, soil organic matter and mineral compositions (Yu et al., 2016; Higgins et al., 2018). Regional verification based on microbial communities has been conducted on shea fruits (El Sheikha et al., 2011), peaches (Bigot et al., 2015) and grapes (Mezzasalma et al., 2017; Mezzasalma et al., 2018). Recently, a comprehensive review summarized the complex relationships between wine quality, grape microbial communities and regional climates (Droby and Wisniewski, 2018). The natural microbiomes of grapes are important for fruit maturation and wine fermentation, especially regarding the formation of some secondary metabolites related to wine colour and flavour (Mezzasalma et al., 2017). Agricultural inputs and farming practices such as fertilizers and pesticides influence the formation of microbial communities on agricultural products (Allard et al., 2018; Chen et al., 2018). Fertilization has been reported to be a factor affecting the plant microbiomes of maize plants (Chen et al., 2018). The products and soil associated with organic and conventional farming practices show differences in their microbiome communities. Specifically, F & V such as grapes (Mezzasalma et al., 2017), apples (Abdelfattah et al., 2016), peaches (Bigot et al., 2015), lettuce and spinach (Leff and Fierer, 2013) produced from organic and conventional agriculture systems have been observed to exhibit differences in their microbial communities. However, the significant relationships between microbiomes and the regional environment and farming practices are only just beginning to be revealed.
Fig. 8.
Microbiomes related to regional/environmental factors and farming practices (A). Microbiomes related to postharvest processing and storage (B).
Effects of artificial treatments in storage and processing on microbiomes
The treatments applied to F & V during postharvest processing and storage affect microbial communities (Fig. 8B). Refrigerated storage is a common approach that is considered to keep food fresh and nutritious. During cold storage, the microbial composition and activity are altered. Variations in microbial communities during cold storage have been reported in apples (Shen et al., 2018a), lettuce and spinach (Lopez‐Velasco et al., 2011). Different fungal structures have been identified between harvest‐point and stored samples (Shen et al., 2018a). Compared with the harvest‐point samples, stored apples show increases in the relative abundance of several genera, particularly Aspergillus, Botrytis, Mucor and Penicillium. The spinach phyllosphere was observed to present a decrease in bacterial diversity after 15 days of storage at 4°C or 10°C, which might be related to the inhibition of bacterial activity by the lower temperature (Lopez‐Velasco et al., 2011). In addition, physical and chemical treatments affect the microbial communities of postharvest F & V. Physical gamma irradiation treatment of romaine lettuce alters the leaf bacterial community and reduces the survival rate and regrowth ability of pathogenic bacteria (Dharmarha et al., 2019a). High‐hydrostatic pressure treatments change the dynamics of the overall bacterial population and extend the shelf life of sweet cherries and asparagus (del Arbol et al., 2016a, b). Antibiotics and fungicides have been shown to alter the microbial communities and extend the shelf life of several F & V. Hypochlorite treatment alters the structure of bacterial communities and extends the shelf life of carrots (Dharmarha et al., 2019b). The application of fungicides alters the dynamics of yeast communities on grape berries (Milanovic et al., 2013). To improve postharvest storage, the relationships between the postharvest treatment of F & V and their microbial communities require more attention.
Conclusion
The development of DNA sequencing technology has provided an effective method for microbial WGS and genetic analysis. In recent decades, DNA sequencing technologies have been successfully developed including approximately ten operational platforms. By performing DNA sequencing, microbial WGS is largely promoted. Genetic studies provide DNA markers for microbial identification at the genetic level. These markers are extensively used in PCR or chip‐based detection. On the basis of 16S rDNA and ITS sequencing, metagenomic approaches are now emerging technologies for analysing the entire microbial community in a complex F & V matrix. The microbiomes of F & V show huge differences between plant species/genotypes. In addition, the microbiomes of F & V are related to factors such as regional/environmental factors, farming practices and postharvest treatments. These studies shed light on ways to improve F & V cultivation, disease prevention and quality control.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We acknowledge Liu Shutong from Shanghai Personal Biotechnology (Shanghai, China) for the help in revised the manuscript.
Microbial Biotechnology (2021) 14(2), 323–362
Funding information
This work was supported by the Earmarked Fund for China Agriculture Research System (No. CARS‐27), the National Program for Quality and Safety Risk Assessment of Agricultural Products of China (No. GJFP2017003, GJFP2018003), and the Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (No. CAAS‐ASTIP).
References
- Abdelfattah, A. , Wisniewski, M. , Droby, S. , and Schena, L. (2016) Spatial and compositional variation in the fungal communities of organic and conventionally grown apple fruit at the consumer point‐of‐purchase. Horticult Res 3: 16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Wahhab, M.A. , Aljawish, A. , Kenawy, A.M. , El‐Nekeety, A.A. , Hamed, H.S. , and Abdel‐Aziem, S.H. (2016) Grafting of gallic acid onto chitosan nano particles enhances antioxidant activities in vitro and protects against ochratoxin A toxicity in catfish (Clarias gariepinus). Environ Toxicol Pharmacol 41: 279–288. [DOI] [PubMed] [Google Scholar]
- Agbaje, M. , Begum, R.H. , Oyekunle, M.A. , Ojo, O.E. , and Adenubi, O.T. (2011) Evolution of Salmonella nomenclature: a critical note. Folia Microbiol 56: 497–503. [DOI] [PubMed] [Google Scholar]
- Aguilar‐Pontes, M.V. , Brandl, J. , McDonnell, E. , Strasser, K. , Nguyen, T.T.M. , Riley, R. , et al. (2018) The gold‐standard genome of Aspergillus niger NRRL 3 enables a detailed view of the diversity of sugar catabolism in fungi. Stud Mycol 91: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu, H. , Taga, M. , Kodama, M. , Johnson, R. , Otani, H. , and Kohmoto, K. (1999) Molecular karyotypes for Alternaria plant pathogens known to produce host‐specific toxins. Curr Genet 35: 647–656. [DOI] [PubMed] [Google Scholar]
- Allard, S.M. , Ottesen, A.R. , Brown, E.W. , and Micallef, S.A. (2018) Insect exclusion limits variation in bacterial microbiomes of tomato flowers and fruit. J Appl Microbiol 125: 1749–1760. [DOI] [PubMed] [Google Scholar]
- Al‐Shuhaib, M.B.S. , Albakri, A.H. , Alwan, S.H. , Almandil, N.B. , AbdulAzeez, S. , and Borgio, J.F. (2018) Optimal pcr primers for rapid and accurate detection of Aspergillus flavus isolates. Microb Pathog 116: 351–355. [DOI] [PubMed] [Google Scholar]
- Alzwghaibi, A.B. , Yahyaraeyat, R. , Fasaei, B.N. , Langeroudi, A.G. , and Salehi, T.Z. (2018) Rapid molecular identification and differentiation of common Salmonella serovars isolated from poultry, domestic animals and foodstuff using multiplex PCR assay. Arch Microbiol 200: 1009–1016. [DOI] [PubMed] [Google Scholar]
- Amin, H.S. , Abdelrahman, A.A. , and Abdellrazeq, G.S. (2016) Occurrence of multidrug‐resistant Salmonella enterica in retail chicken meat and development of a six genes‐based multiplex PCR as an alternative diagnostic method. J Food Safety 36: 459–466. [Google Scholar]
- Amselem, J. , Cuomo, C.A. , van Kan, J.A. , Viaud, M. , Benito, E.P. , Couloux, A. , et al. (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7: e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, B. , Smedsgaard, J. , Jorring, I. , Skouboe, P. , and Pedersen, L.H. (2006) Real‐time PCR quantification of the AM‐toxin gene and HPLC qualification of toxigenic metabolites from Alternaria species from apples. Int J Food Microbiol 111: 105–111. [DOI] [PubMed] [Google Scholar]
- del Arbol, J.T. , Pulido, R.P. , La Storia, A. , Burgos, M.J.G. , Lucas, R. , Ercolini, D. , et al. (2016a) Changes in microbial diversity of brined green asparagus upon treatment with high hydrostatic pressure. Int J Food Microbiol 216: 1–8. [DOI] [PubMed] [Google Scholar]
- del Arbol, J.T. , Pulido, R.P. , La Storia, A. , Burgos, M.J.G. , Lucas, R. , Ercolini, D. , et al. (2016b) Microbial diversity in pitted sweet cherries (Prunus avium L.) as affected by High‐Hydrostatic Pressure treatment. Food Res Int 89: 790–796. [DOI] [PubMed] [Google Scholar]
- Atoui, A. , and El Khoury, A. (2017) PCR‐RFLP for Aspergillus species. Methods Mol Biol 1542: 313–320. [DOI] [PubMed] [Google Scholar]
- Atoui, A. , El Khoury, A. , Kallassy, M. , and Lebrihi, A. (2012) Quantification of Fusarium graminearum and Fusarium culmorum by real‐time PCR system and zearalenone assessment in maize. Int J Food Microbiol 154: 59–65. [DOI] [PubMed] [Google Scholar]
- Ayukawa, Y. , Komatsu, K. , Kashiwa, T. , Akai, K. , Yamada, M. , Teraoka, T. , et al. (2016) Detection and differentiation of Fusarium oxysporum f. sp. lycopersici race 1 using loop‐mediated isothermal amplification with three primer sets. Lett Appl Microbiol 63: 202–209. [DOI] [PubMed] [Google Scholar]
- Ballester, A.R. , Marcet‐Houben, M. , Levin, E. , Sela, N. , Selma‐Lazaro, C. , Carmona, L. , et al. (2015) Genome, transcriptome, and functional analyses of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol Plant Microbe Interact 28: 232–248. [DOI] [PubMed] [Google Scholar]
- Bankier, A.T. , Beck, S. , Bohni, R. , Brown, C.M. , Cerny, R. , Chee, M.S. , et al. (1991) The DNA sequence of the human cytomegalovirus genome. DNA Sequence 2: 1–11. [DOI] [PubMed] [Google Scholar]
- Banowary, B. , Dang, V.T. , Sarker, S. , Connolly, J.H. , Chenu, J. , Groves, P. , et al. (2018) Evaluation of two multiplex PCR‐high‐resolution melt curve analysis methods for differentiation of Campylobacter jejuni and Campylobacter coli intraspecies. Avian Dis 62: 86–93. [DOI] [PubMed] [Google Scholar]
- Basim, H. , Basim, E. , Baki, D. , Abdulai, M. , Ozturk, N. , and Balkic, R. (2018) Identification and characterization of Alternaria alternata (Fr.) Keissler causing Ceratonia Blight disease of carob (Ceratonia siliqua L.) in Turkey. Eur J Plant Pathol 151: 73–86. [Google Scholar]
- Baysal‐Gurel, F. , Subedi, N. , Mamiro, D.P. , and Miller, S.A. (2014) First report of Anthracnose of onion caused by Colletotrichum coccodes in Ohio. Plant Dis 98: 1271. [DOI] [PubMed] [Google Scholar]
- van den Beld, M.J.C. , and Reubsaet, F.A.G. (2012) Differentiation between Shigella, enteroinvasive Escherichia coli (EIEC) and noninvasive Escherichia coli . Eur J Clin Microbiol Infect Dis 31: 899–904. [DOI] [PubMed] [Google Scholar]
- van den Berg, M.A. , Albang, R. , Albermann, K. , Badger, J.H. , Daran, J.M. , Driessen, A.J.M. , et al. (2008) Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum . Nat Biotechnol 26: 1161–1168. [DOI] [PubMed] [Google Scholar]
- de Biazio, G.R. , Leite, G.G. , Tessmann, D.J. , and Barbosa‐Tessmann, I.P. (2008) A new PCR approach for the identification of Fusarium graminearum . Braz J Microbiol 39: 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, M. , Matsuzawa, T. , Sakai, K. , Muraosa, Y. , Lyra, L. , Busso‐Lopes, A.F. , et al. (2017) Comparison of DNA Microarray, Loop‐Mediated Isothermal Amplification (LAMP) and Real‐Time PCR with DNA sequencing for identification of Fusarium spp. obtained from patients with hematologic malignancies. Mycopathologia 182: 625–632. [DOI] [PubMed] [Google Scholar]
- Bigot, C. , Meile, J.‐C. , Kapitan, A. , and Montet, D. (2015) Discriminating organic and conventional foods by analysis of their microbial ecology: an application on fruits. Food Control 48: 123–129. [Google Scholar]
- Blattner, F.R. , Plunkett, G. 3rd , Bloch, C.A. , Perna, N.T. , Burland, V. , Riley, M. , et al. (1997) The complete genome sequence of Escherichia coli K‐12. Science 277: 1453–1462. [DOI] [PubMed] [Google Scholar]
- Bokulich, N.A. , Thorngate, J.H. , Richardson, P.M. , and Mills, D.A. (2014) Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci USA 111: E139–E148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego‐Benjumea, A. , Basallote‐Ureba, M.J. , Melero‐Vara, J.M. , and Abbasi, P.A. (2014) Characterization of Fusarium isolates from asparagus fields in southwestern Ontario and influence of soil organic amendments on Fusarium crown and root rot. Phytopathology 104: 403–415. [DOI] [PubMed] [Google Scholar]
- Brandwein, M. , Al‐Quntar, A. , Goldberg, H. , Mosheyev, G. , Goffer, M. , Marin‐Iniesta, F. , et al. (2016) Mitigation of biofilm formation on corrugated cardboard fresh produce packaging surfaces using a novel thiazolidinedione derivative integrated in acrylic emulsion polymers. Front Microbiol 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, R.L. , Gorris, L.G.M. , Hayman, M.M. , Jackson, T.C. , and Whiting, R.C. (2017) A review of Listeria monocytogenes: an update on outbreaks, virulence, dose‐response, ecology, and risk assessments. Food Control 75: 1–13. [Google Scholar]
- Callicott, K.A. , and Cotty, P.J. (2015) Method for monitoring deletions in the aflatoxin biosynthesis gene cluster of Aspergillus flavus with multiplex PCR. Lett Appl Microbiol 60: 60–65. [DOI] [PubMed] [Google Scholar]
- Campos‐Pena, E. , Martin‐Nunez, E. , Pulido‐Reyes, G. , Martin‐Padron, J. , Caro‐Carrillo, E. , Donate‐Correa, J. , et al. (2014) Multiplex PCR assay for identification of six different Staphylococcus spp. and simultaneous detection of methicillin and mupirocin resistance. J Clin Microbiol 52: 2698–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano, J. , Guarro, J. , and Gene, J. (2004) Molecular and morphological identification of Colletotrichum species of clinical interest. J Clin Microbiol 42: 2450–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Fanning, S. , Proos, S. , Jordan, K. , and Srikumar, S. (2017) A review on the applications of next generation sequencing technologies as applied to food‐related microbiome studies. Front Microbiol 8: 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F.D. , Costello, E.K. , et al. (2010) QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro, G.A. , Matic, S. , Ortu, G. , Garibaldi, A. , Spadaro, D. , and Gullino, M.L. (2017) Development and validation of a TaqMan Real‐Time PCR assay for the specific detection and quantification of Fusarium fujikuroi in rice plants and seeds. Phytopathology 107: 885–892. [DOI] [PubMed] [Google Scholar]
- Casasnovas, F. , Fantini, E.N. , Palazzini, J.M. , Giaj‐Merlera, G. , Chulze, S.N. , Reynoso, M.M. , et al. (2013) Development of amplified fragment length polymorphism (AFLP)‐derived specific primer for the detection of Fusarium solani aetiological agent of peanut brown root rot. J Appl Microbiol 114: 1782–1792. [DOI] [PubMed] [Google Scholar]
- Cernava, T. , Erlacher, A. , Soh, J. , Sensen, C.W. , Grube, M. , and Berg, G. (2019) Enterobacteriaceae dominate the core microbiome and contribute to the resistome of arugula (Eruca sativa Mill.). Microbiome 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens, S. , Delbeke, S. , De Coninck, D. , Boussemaere, J. , Boon, N. , and Uyttendaele, M. (2015) Characterization of the bacterial community naturally present on commercially grown basil leaves: evaluation of sample preparation prior to culture‐independent techniques. Int J Environ Res Public Health 12: 10171–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.S. , Tsay, J.G. , Huang, Y.F. , and Chiou, R.Y. (2002) Polymerase chain reaction‐mediated characterization of molds belonging to the Aspergillus flavus group and detection of Aspergillus parasiticus in peanut kernels by a multiplex polymerase chain reaction. J Food Prot 65: 840–844. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Wen, X. , Sun, Y. , Zhang, J. , Lin, X. , and Liao, Y. (2015) Effect of ground mulch managements on soil bacterial community structure and diversity in the non‐irrigated apple orchard in Weibei Loess Plateau. Acta Microbiol Sin 55: 892–904. [PubMed] [Google Scholar]
- Chen, J. , Wu, L. , Xiao, Z. , Wu, Y. , Wu, H. , Qin, X. , et al. (2017) Assessment of the diversity of Pseudomonas spp. and Fusarium spp. in Radix pseudostellariae rhizosphere under monoculture by combining DGGE and quantitative PCR. Front Microbiol 8: 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Tian, Z. , Wang, L. , and Long, C.A. (2017) Development of specific primers based on the genomes of Penicillium spp. for rapid detection of Penicillium digitatum among fungal isolates in citrus. Eur J Plant Pathol 149: 201–209. [Google Scholar]
- Chen, Q.L. , An, X.L. , Zheng, B.X. , Ma, Y.B. , and Su, J.Q. (2018) Long‐term organic fertilization increased antibiotic resistome in phyllosphere of maize. Sci Total Environ 645: 1230–1237. [DOI] [PubMed] [Google Scholar]
- Chen, K. , Tian, Z. , Jiang, F. , and Long, C.A. (2019) Development of Penicillium italicum‐specific primers for rapid detection among fungal isolates in citrus. J Microbiol Biotechnol 29: 984–988. [DOI] [PubMed] [Google Scholar]
- Chitrampalam, P. , Abraham, N. , and Nelson, B.D. Jr (2018) A culture‐independent PCR‐based assay to detect the root rot pathogen Fusarium solani species complex 11 from soybean roots and soil. Plant Dis 102: 327–333. [DOI] [PubMed] [Google Scholar]
- Chou, M.Y. , Vanden Heuvel, J. , Bell, T.H. , Panke‐Buisse, K. , and Kao‐Kniffin, J. (2018) Vineyard under‐vine floor management alters soil microbial composition, while the fruit microbiome shows no corresponding shifts. Sci Rep 8: 11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, W.H. , Chung, W.C. , Peng, M.T. , Yang, H.R. , and Huang, J.W. (2010) Specific detection of benzimidazole resistance in Colletotrichum gloeosporioides from fruit crops by PCR‐RFLP. New Biotechnol 27: 17–24. [DOI] [PubMed] [Google Scholar]
- Coleman, J.J. , Rounsley, S.D. , Rodriguez‐Carres, M. , Kuo, A. , Wasmann, C.C. , Grimwood, J. , et al. (2009) The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genet 5: e1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, M.J. , Tardif, M.C. , and Meldrum, A.J. (2004) Identification of Monilinia fructigena, M. fructicola, M. laxa, and Monilia polystroma on inoculated and naturally infected fruit using multiplex PCR. Plant Dis 88: 1219–1225. [DOI] [PubMed] [Google Scholar]
- Cuomo, C.A. , Guldener, U. , Xu, J.R. , Trail, F. , Turgeon, B.G. , Di Pietro, A. , et al. (2007) The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317: 1400–1402. [DOI] [PubMed] [Google Scholar]
- Dalmasso, A. , Rantsiou, K. , Cocolin, L. , and Bottero, M.T. (2010) Development of a biomolecular assay for the identification of Listeria at species level. Foodborne Pathog Dis 7: 565–571. [DOI] [PubMed] [Google Scholar]
- van Dam, P. , de Sain, M. , Ter Horst, A. , van der Gragt, M. , and Rep, M. (2018) Use of comparative genomics‐based markers for discrimination of host specificity in Fusarium oxysporum . Appl Environ Microbiol 84: e01868‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, E.L. , Auger, H. , Jaszczyszyn, Y. , and Thermes, C. (2014) Ten years of next‐generation sequencing technology. Trends Genet 30: 418–426. [DOI] [PubMed] [Google Scholar]
- van Dijk, E.L. , Jaszczyszyn, Y. , Naquin, D. , and Thermes, C. (2018) The third revolution in sequencing technology. Trends Genet 34: 666–681. [DOI] [PubMed] [Google Scholar]
- Darlison, J. , Mogren, L. , Rosberg, A.K. , Gruden, M. , Minet, A. , Line, C. , et al. (2019) Leaf mineral content govern microbial community structure in the phyllosphere of spinach (Spinacia oleracea) and rocket (Diplotaxis tenuifolia). Sci Total Environ 675: 501–512. [DOI] [PubMed] [Google Scholar]
- Das, P. , Pandey, P. , Harishankar, A. , Chandy, M. , Bhattacharya, S. , and Chakrabarti, A. (2017) Standardization of a two‐step real‐time polymerase chain reaction based method for species‐specific detection of medically important Aspergillus species. Indian J Med Microbiol 35: 381–388. [DOI] [PubMed] [Google Scholar]
- Dauch, A.L. , Watson, A.K. , and Jabaji‐Hare, S.H. (2003) Detection of the biocontrol agent Colletotrichum coccodes (183088) from the target weed velvetleaf and from soil by strain‐specific PCR markers. J Microbiol Methods 55: 51–64. [DOI] [PubMed] [Google Scholar]
- De, C.N. , Vlaemynck, G. , Van, P.E. , Van, W.S. , Herman, L. , Devlieghere, F. , et al. (2016) Isoepoxydon dehydrogenase (idh) gene expression in relation to patulin production by Penicillium expansum under different temperature and atmosphere. Int J Food Microbiol 220: 50–57. [DOI] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A.L. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , et al. (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13: 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbi, A. , Boureghda, H. , Monte, E. , and Hermosa, R. (2018) Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in Algeria and a biocontrol strategy with indigenous Trichoderma spp. Front Microbiol 9: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debode, J. , De Tender, C. , Soltaninejad, S. , Van Malderghem, C. , Haegeman, A. , Van der Linden, I. , et al. (2016) Chitin mixed in potting soil alters lettuce growth, the survival of zoonotic bacteria on the leaves and associated rhizosphere microbiology. Front Microbiol 7: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degola, F. , Berni, E. , Dall'Asta, C. , Spotti, E. , Marchelli, R. , Ferrero, I. , et al. (2007) A multiplex RT‐PCR approach to detect aflatoxigenic strains of Aspergillus flavus . J Appl Microbiol 103: 409–417. [DOI] [PubMed] [Google Scholar]
- Demeke, T. , Grafenhan, T. , Clear, R.M. , Phan, A. , Ratnayaka, I. , Chapados, J. , et al. (2010) Development of a specific TaqMan real‐time PCR assay for quantification of Fusarium graminearum clade 7 and comparison of fungal biomass determined by PCR with deoxynivalenol content in wheat and barley. Int J Food Microbiol 141: 45–50. [DOI] [PubMed] [Google Scholar]
- Deng, B. , Wang, W.H. , Deng, L.L. , Yao, S.X. , Ming, J. , and Zeng, K.F. (2018) Comparative RNA‐seq analysis of citrus fruit in response to infection with three major postharvest fungi. Postharvest Biol Technol 146: 134–146. [Google Scholar]
- Desai, P.T. , Porwollik, S. , Long, F. , Cheng, P. , Wollam, A. , Clifton, S.W. , et al. (2013) Evolutionary genomics of Salmonella enterica subspecies. Mbio 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarha, V. , Guron, G. , Boyer, R.R. , Niemira, B.A. , Pruden, A. , Strawn, L.K. , et al. (2019a) Gamma irradiation influences the survival and regrowth of antibiotic‐resistant bacteria and antibiotic‐resistance genes on romaine lettuce. Front Microbiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarha, V. , Pulido, N. , Boyer, R.R. , Pruden, A. , Strawn, L.K. , and Ponder, M.A. (2019b) Effect of post‐harvest interventions on surficial carrot bacterial community dynamics, pathogen survival, and antibiotic resistance. Int J Food Microbiol 291: 25–34. [DOI] [PubMed] [Google Scholar]
- Diguta, C.F. , Rousseaux, S. , Weidmann, S. , Bretin, N. , Vincent, B. , Guilloux‐Benatier, M. , et al. (2010) Development of a qPCR assay for specific quantification of Botrytis cinerea on grapes. FEMS Microbiol Lett 313: 81–87. [DOI] [PubMed] [Google Scholar]
- Ding, N. , Xing, F. , Liu, X. , Selvaraj, J.N. , Wang, L. , Zhao, Y. , et al. (2015). Variation in fungal microbiome (mycobiome) and aflatoxin in stored in‐shell peanuts at four different areas of China. Front Microbiol 6: 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell, N. , Mangan, J.A. , Laing, K.G. , Hinds, J. , Linton, D. , Al‐Ghusein, H. , et al. (2001) Whole genome comparison of Campylobacter jejuni human isolates using a low‐cost microarray reveals extensive genetic diversity. Genome Res 11: 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt, M. , and Raoult, D. (2002) rpoB gene sequence‐based identification of Staphylococcus species. J Clin Microbiol 40: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droby, S. , and Wisniewski, M. (2018) The fruit microbiome: a new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol Technol 140: 107–112. [Google Scholar]
- Dufour, P. , Jarraud, S. , Vandenesch, F. , Greenland, T. , Novick, R.P. , Bes, M. , et al. (2002) High genetic variability of the agr locus in Staphylococcus species. J Bacteriol 184: 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand, N. , Fontana, A. , Meile, J.‐C. , Suarez‐Quiroz, M.‐L. , Schorr‐Galindo, S. , and Montet, D. (2019) Differentiation and quantification of the ochratoxin A producers Aspergillus ochraceus and Aspergillus westerdijkiae using PCR‐DGGE. J Basic Microbiol 59: 158–165. [DOI] [PubMed] [Google Scholar]
- Dutta, S. , Chatterjee, A. , Dutta, P. , Rajendran, K. , Roy, S. , Pramanik, K.C. , et al. (2001) Sensitivity and performance characteristics of a direct PCR with stool samples in comparison to conventional techniques for diagnosis of Shigella and enteroinvasive Escherichia coli infection in children with acute diarrhoea in Calcutta, India. J Med Microbiol 50: 667–674. [DOI] [PubMed] [Google Scholar]
- Duvenage, F.J. , Duvenage, S. , Du Plessis, E.M. , Volschenk, Q. , and Korsten, L. (2017) Viable bacterial population and persistence of foodborne pathogens on the pear carpoplane. J Sci Food Agric 97: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Einson, J.E. , Rani, A. , You, X.M. , Rodriguez, A.A. , Randell, C.L. , Barnaba, T. , et al. (2018) A vegetable fermentation facility hosts distinct microbiomes reflecting the production environment. Appl Environ Microbiol 84: e01680‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury, R. , Atoui, A. , Verheecke, C. , Maroun, R. , El Khoury, A. , and Mathieu, F. (2016) Essential oils modulate gene expression and Ochratoxin A production in Aspergillus carbonarius . Toxins 8: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sheikha, A.F. , Bouvet, J.M. , and Montet, D. (2011) Biological bar code for determining the geographical origin of fruits using 285 rDNA fingerprinting of fungal communities by PCR‐DGGE: an application to Shea tree fruits. Quality Assurance Safety Crops Foods 3: 40–47. [Google Scholar]
- Elfar, K. , Zoffoli, J.P. , and Latorre, B.A. (2018) Identification and characterization of Alternaria species associated with Moldy Core of apple in Chile. Plant Dis 102: 2158–2169. [DOI] [PubMed] [Google Scholar]
- Erlacher, A. , Cardinale, M. , Grube, M. , and Berg, G. (2015) Biotic stress shifted structure and abundance of Enterobacteriaceae in the lettuce microbiome. Plos One 10: e0118068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahati, M. , Ghojoghi, A. , Abastabar, M. , Ghasemi, Z. , Farahyar, S. , Roudbary, M. , et al. (2016) The first case of total dystrophic Onychomycosis caused by Aspergillus clavatus resistant to antifungal drugs. Mycopathologia 181: 273–277. [DOI] [PubMed] [Google Scholar]
- Fan, J. , Luo, Y. , Michailides, T.J. , and Guo, L. (2014) Simultaneous quantification of alleles E198A and H6Y in the beta‐tubulin gene conferring benzimidazole resistance in Monilinia fructicola using a duplex real‐time (TaqMan) PCR. Pest Manag Sci 70: 245–251. [DOI] [PubMed] [Google Scholar]
- Fan, X. , Zhang, J. , Yang, L. , Wu, M. , Chen, W. , and Li, G. (2015) Development of PCR‐based assays for detecting and differentiating three species of botrytis infecting broad bean. Plant Dis 99: 691–698. [DOI] [PubMed] [Google Scholar]
- Farfan, M.J. , Garay, T.A. , Prado, C.A. , Filliol, I. , Ulloa, M.T. , and Toro, C.S. (2010) A new multiplex PCR for differential identification of Shigella flexneri and Shigella sonnei and detection of Shigella virulence determinants. Epidemiol Infect 138: 525–533. [DOI] [PubMed] [Google Scholar]
- Faria, C.B. , Abe, C.A. , da Silva, C.N. , Tessmann, D.J. , and Barbosa‐Tessmann, I.P. (2012) New PCR assays for the identification of Fusarium verticillioides, Fusarium subglutinans, and other species of the Gibberella fujikuroi complex. Int J Mol Sci 13: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshad, S. , Ranjbar, R. , and Hosseini, M. (2015) Molecular genotyping of Shigella sonnei strains isolated from children with bloody diarrhea using pulsed field gel electrophoresis on the total genome and PCR‐RFLP of IpaH and IpaBCD genes. Jundishapur J Microbiol 8: e14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustinelli, P.C. , Wang, X.M. , Palencia, E.R. , and Arias, R.S. (2016) Genome sequences of eight Aspergillus flavus spp. and one A. parasiticus sp., isolated from peanut seeds in Georgia. Genome Announc 4: e00278‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas, M. , Madar, A. , Sipiczki, M. , Miklos, I. , and Holb, I.J. (2014) Genetic diversity in Monilinia laxa populations in stone fruit species in Hungary. World J Microbiol Biotechnol 30: 1879–1892. [DOI] [PubMed] [Google Scholar]
- Fiedoruk, K. , Daniluk, T. , Swiecicka, I. , Murawska, E. , Sciepuk, M. , and Leszczynska, K. (2014) First complete genome sequence of Escherichia albertii strain KF1, a new potential human enteric pathogen. Genome Announc 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann, R.D. , Adams, M.D. , White, O. , Clayton, R.A. , Kirkness, E.F. , Kerlavage, A.R. , et al. (1995) Whole‐genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269: 496–512. [DOI] [PubMed] [Google Scholar]
- Foley, S.L. , Lynne, A.M. , and Nayak, R. (2009) Molecular typing methodologies for microbial source tracking and epidemiological investigations of Gram‐negative bacterial foodborne pathogens. Infect Genet Evol 9: 430–440. [DOI] [PubMed] [Google Scholar]
- Forbes, J.D. , Knox, N.C. , Ronholm, J. , Pagotto, F. , and Reimer, A. (2017) Metagenomics: the next culture‐independent game changer. Front Microbiol 8: 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds, I.V. , Granacki, A. , Xiao, C. , Krull, U.J. , Castle, A. , and Horgen, P.A. (2002) Quantification of microcystin‐producing cyanobacteria and E‐coli in water by 5′‐nuclease PCR. J Appl Microbiol 93: 825–834. [DOI] [PubMed] [Google Scholar]
- Franz, E. , Klerks, M.A. , De Vos, O.J. , Termorshuizen, A.J. , and van Bruggen, A.H.C. (2007) Prevalence of Shiga toxin‐producing Escherichia coli stx(1), stx(2), eaeA, and rfbE genes and survival of E‐coli O157: H7 in manure from organic and low‐input conventional dairy farms. Appl Environ Microbiol 73: 2180–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasao, B.D. , Marin, V.A. , and Conte, C.A. (2017) Molecular detection, typing, and quantification of Campylobacter spp. in foods of animal origin. Comp Rev Food Sci Food Safety 16: 721–734. [DOI] [PubMed] [Google Scholar]
- Frebourg, N.B. , Lefebvre, S. , Baert, S. , and Lemeland, J.F. (2000) PCR‐based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J Clin Microbiol 38: 877–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, C.E. , and Brown, A.E. (1997) Use of SSU rDNA group‐I intron to distinguish Monilinia fructicola from M. laxa and M. fructigena . FEMS Microbiol Lett 157: 307–312. [DOI] [PubMed] [Google Scholar]
- Furuta, K. , Nagashima, S. , Inukai, T. , and Masuta, C. (2017) Construction of a system for the Strawberry Nursery Production towards elimination of latent infection of Anthracnose fungi by a combination of PCR and microtube hybridization. Plant Pathol J 33: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, M.F. , Uriel, N. , Teifoori, F. , Postigo, I. , Sunen, E. , and Martinez, J. (2017) The major Alternaria alternata allergen, Alt a 1: A reliable and specific marker of fungal contamination in citrus fruits. Int J Food Microbiol 257: 26–30. [DOI] [PubMed] [Google Scholar]
- Gachon, C. , and Saindrenan, P. (2004) Real‐time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea . Plant Physiol Biochem 42: 367–371. [DOI] [PubMed] [Google Scholar]
- Gai, X. , Dong, H. , Wang, S. , Liu, B. , Zhang, Z. , Li, X. , et al. (2018) Infection cycle of maize stalk rot and ear rot caused by Fusarium verticillioides. PLoS One 13: e0201588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez, L. , Urbaniak, M. , Waskiewicz, A. , Stepien, L. , and Palmero, D. (2017) Fusarium proliferatum – causal agent of garlic bulb rot in Spain: genetic variability and mycotoxin production. Food Microbiol 67: 41–48. [DOI] [PubMed] [Google Scholar]
- Gan, P. , Nakata, N. , Suzuki, T. , and Shirasu, K. (2017) Markers to differentiate species of anthracnose fungi identify Colletotrichum fructicola as the predominant virulent species in strawberry plants in Chiba Prefecture of Japan. J Gen Plant Pathol 83: 14–22. [Google Scholar]
- Gannon, V.P.J. , Dsouza, S. , Graham, T. , King, R.K. , Rahn, K. , and Read, S. (1997) Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol 35: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , Liu, Y.N. , Nan, N. , Lu, B.H. , Xia, W.Y. , and Wu, X.Y. (2014) Alternaria brassicicola causes a leaf spot on Isatis indigotica in China. Plant Dis 98: 1431. [DOI] [PubMed] [Google Scholar]
- Garganese, F. , Schena, L. , Siciliano, I. , Prigigallo, M.I. , Spadaro, D. , De Grassi, A. , et al. (2016) Characterization of Citrus‐associated Alternaria species in Mediterranean Areas. PLoS One 11: e0163255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia, G. , Umpierrez‐Failache, M. , Ward, T.J. , and Vero, S. (2018) Development of a PCR‐RFLP method based on the transcription elongation factor 1‐alpha gene to differentiate Fusarium graminearum from other species within the Fusarium graminearum species complex. Food Microbiol 70: 28–32. [DOI] [PubMed] [Google Scholar]
- Gasanov, U. , Hughes, D. , and Hansbro, P.M. (2005) Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: a review. FEMS Microbiol Rev 29: 851–875. [DOI] [PubMed] [Google Scholar]
- Gaszewska‐Mastalarz, A. , Zakrzewska‐Czerwinska, J. , and Mordarski, M. (1997) Rapid detection of Staphylococcus saprophyticus using primer specific PCR. Acta Biol Hung 48: 319–322. [PubMed] [Google Scholar]
- Ghanbari, M. , Kneifel, W. , and Domig, K.J. (2015) A new view of the fish gut microbiome: advances from next‐generation sequencing. Aquaculture 448: 464–475. [Google Scholar]
- Gherbawy, Y. , Hussein, M.A. , Runge, F. , and Spring, O. (2018) Molecular characterization of Alternaria alternata population isolated from Upper Egyptian tomato fruits. J Phytopathol 166: 709–721. [Google Scholar]
- Ghorbanalizadgan, M. , Bakhshi, B. , Kazemnejad Lili, A. , Najar‐Peerayeh, S. , and Nikmanesh, B. (2014) A molecular survey of Campylobacter jejuni and Campylobacter coli virulence and diversity. Iran Biomed J 18: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaj Merlera, G. , Munoz, S. , Coelho, I. , Cavaglieri, L.R. , Torres, A.M. , and Reynoso, M.M. (2015) Diversity of black Aspergilli isolated from raisins in Argentina: polyphasic approach to species identification and development of SCAR markers for Aspergillus ibericus . Int J Food Microbiol 210: 92–101. [DOI] [PubMed] [Google Scholar]
- Gillespie, I.A. , O'Brien, S.J. , Frost, J.A. , Adak, G.K. , Peter, H. , Swan, A.V. , et al. (2002) A case‐case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg Infect Dis 8: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Serna, J. , Vázquez, C. , Sardiñas, N. , González‐Jaén, M.T. , and Patiño, B. (2009) Discrimination of the main Ochratoxin A‐producing species in Aspergillus section Circumdati by specific PCR assays. Int J Food Microbiol 136: 83–87. [DOI] [PubMed] [Google Scholar]
- Gil‐Serna, J. , Patino, B. , Cortes, L. , Teresa Gonzalez‐Jaen, M. , and Vazquez, C. (2011) Mechanisms involved in reduction of ochratoxin A produced by Aspergillus westerdijkiae using Debaryomyces hansenii CYC 1244. Int J Food Microbiol 151: 113–118. [DOI] [PubMed] [Google Scholar]
- Glaser, P. , Frangeul, L. , Buchrieser, C. , Rusniok, C. , Amend, A. , Baquero, F. , et al. (2001) Comparative genomics of Listeria species. Science 294: 849–852. [DOI] [PubMed] [Google Scholar]
- Goebel, S.J. , Johnson, G.P. , Perkus, M.E. , Davis, S.W. , Winslow, J.P. , and Paoletti, E. (1990) The complete DNA sequence of vaccinia virus. Virology 179: 247–266. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Salgado, A. , Patino, B. , Vazquez, C. , and Gonzalez‐Jaen, M.T. (2005) Discrimination of Aspergillus niger and other Aspergillus species belonging to section Nigri by PCR assays. FEMS Microbiol Lett 245: 353–361. [DOI] [PubMed] [Google Scholar]
- Grillova, L. , Sedlacek, I. , Pachnikova, G. , Stankova, E. , Svec, P. , Holochova, P. , et al. (2018) Characterization of four Escherichia albertii isolates collected from animals living in Antarctica and Patagonia. J Vet Med Sci 80: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont, P.A.D. , and Weill, F.X. (2007) Antigenic Formulae of the Salmonella Serovars: WHO Collaborating Centre for Reference and Research on Salmonella, 9th edn. Paris, France: Institute Pasteur, pp. 1–166. [Google Scholar]
- Gu, Q. , Yang, Z.H. , Zhao, D.M. , Zhang, D. , Wang, Q. , Ma, L.S. , et al. (2017) Development of a semi‐nested PCR‐based method for specific and rapid detection of Alternaria solani causing potato early blight in soil. Curr Microbiol 74: 1083–1088. [DOI] [PubMed] [Google Scholar]
- Guinet, C. , Fourrier‐Jeandel, C. , Cerf‐Wendling, I. , and Ioos, R. (2016) One‐step detection of Monilinia fructicola, M. fructigena, and M. laxa on Prunus and Malus by a multiplex real‐time PCR assay. Plant Dis 100: 2465–2474. [DOI] [PubMed] [Google Scholar]
- Hage, E. , Mpamugo, O. , Ohai, C. , Sapkota, S. , Swift, C. , Wooldridge, D. , et al. (2014) Identification of six Listeria species by real‐time PCR assay. Lett Appl Microbiol 58: 535–540. [DOI] [PubMed] [Google Scholar]
- Hale, T.L. (1991) Genetic basis of virulence in Shigella species. Microbiol Rev 55: 206–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto, H. , Hasegawa, K. , Nakaune, R. , Lee, Y.J. , Akutsu, K. , and Hibi, T. (2001) PCR‐based detection of sterol demethylation inhibitor‐resistant strains of Penicillium digitatum. Pest Management Science 57: 839–843. [DOI] [PubMed] [Google Scholar]
- Hammami, W. , Al‐Thani, R. , Fiori, S. , Al‐Meer, S. , Atia, F.A. , Rabah, D. , et al. (2017) Patulin and patulin producing Penicillium spp. occurrence in apples and apple‐based products including baby food. J Infect Dev Ctries 11: 343–349. [DOI] [PubMed] [Google Scholar]
- Harteveld, D.O.C. , Akinsanmi, O.A. , and Drenth, A. (2014) Pathogenic variation of Alternaria species associated with leaf blotch and fruit spot of apple in Australia. Eur J Plant Pathol 139: 789–799. [Google Scholar]
- Hassan, J. , Awasthi, S.P. , Hatanaka, N. , Okuno, K. , Hoang, P.H. , Nagita, A. , et al. (2018) Development of a multiplex PCR targeting eae, stx and cdt genes in genus Escherichia and detection of a novel cdtB gene in Providencia rustigianii . Pathog Dis 76: ftz002. [DOI] [PubMed] [Google Scholar]
- Hayashi, T. , Makino, K. , Ohnishi, M. , Kurokawa, K. , Ishii, K. , Yokoyama, K. , et al. (2001) Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K‐12. DNA Res 8: 11–22. [DOI] [PubMed] [Google Scholar]
- Heilmann, L.J. , Nitzan, N. , Johnson, D.A. , Pasche, J.S. , Doetkott, C. , and Gudmestad, N.C. (2006) Genetic variability in the potato pathogen colletotrichum coccodes as determined by amplified fragment length polymorphism and vegetative compatibility group analyses. Phytopathology 96: 1097–1107. [DOI] [PubMed] [Google Scholar]
- von Hertwig, A.M. , Sant'Ana, A.S. , Sartori, D. , da Silva, J.J. , Nascimento, M.S. , Iamanaka, B.T. , et al. (2018) Real‐time PCR‐based method for rapid detection of Aspergillus niger and Aspergillus welwitschiae isolated from coffee. J Microbiol Methods 148: 87–92. [DOI] [PubMed] [Google Scholar]
- Hesham, E. , Abdul Aziz, B. , and Youssuf, G. (2011) Genotypic identification of Penicillium expansum and the role of processing on patulin presence in juice. Food Chem Toxicol 49: 941–946. [DOI] [PubMed] [Google Scholar]
- Higgins, D. , Pal, C. , Sulaiman, I.M. , Jia, C.R. , Zerwekh, T. , Dowd, S.E. , et al. (2018) Application of high‐throughput pyrosequencing in the analysis of microbiota of food commodities procured from small and large retail outlets in a US metropolitan area – a pilot study. Food Res Int 105: 29–40. [DOI] [PubMed] [Google Scholar]
- Hirvonen, J.J. (2014) The use of molecular methods for the detection and identification of methicillin‐resistant Staphylococcus aureus . Biomarkers Med 8: 1115–1125. [DOI] [PubMed] [Google Scholar]
- Hsu, W.B. , Wang, J.H. , Chen, P.C. , Lu, Y.S. , and Chen, J.H. (2007) Detecting low concentrations of Shigella sonnei in environmental water samples by PCR. FEMS Microbiol Lett 270: 291–298. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Chen, C. , Peever, T. , Dang, H. , Lawrence, C. , and Mitchell, T. (2012) Genomic characterization of the conditionally dispensable chromosome in Alternaria arborescens provides evidence for horizontal gene transfer. BMC Genomics 13: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, S.S.T. , Palumbo, J.D. , Dan, E.P. , Sarreal, S.B.L. , and O’Keeffe, T.L. (2018) Development of a droplet digital PCR assay for population analysis of aflatoxigenic and atoxigenic Aspergillus flavus mixtures in soil. Mycotoxin Res 34: 187–194. [DOI] [PubMed] [Google Scholar]
- Iorio, N.L. , Azevedo, M.B. , Frazao, V.H. , Barcellos, A.G. , Barros, E.M. , Pereira, E.M. , et al. (2011) Methicillin‐resistant Staphylococcus epidermidis carrying biofilm formation genes: detection of clinical isolates by multiplex PCR. Int Microbiol 14: 13–17. [DOI] [PubMed] [Google Scholar]
- Jarvis, K.G. , Daquigan, N. , White, J.R. , Morin, P.M. , Howard, L.M. , Manetas, J.E. , et al. (2018) Microbiomes associated with foods from plant and animal sources. Front Microbiol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedidi, I. , Soldevilla, C. , Lahouar, A. , Marin, P. , Gonzalez‐Jaen, M.T. , and Said, S. (2018) Mycoflora isolation and molecular characterization of Aspergillus and Fusarium species in Tunisian cereals. Saudi J Biol Sci 25: 868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelev, Z.J. , Bobev, S.G. , Minz, D. , Maymon, M. , and Freeman, S. (2008) First report of Anthracnose fruit rot caused by Colletotrichum acutatum on pepper and tomato in Bulgaria. Plant Dis 92: 172. [DOI] [PubMed] [Google Scholar]
- Jeyasekaran, G. , Raj, K.T. , Shakila, R.J. , Thangarani, A.J. , Sukumar, D. , and Jailani, V.A.K. (2011) Rapid detection of Salmonella enterica serovars by multiplex PCR. World J Microbiol Biotechnol 27: 953–959. [Google Scholar]
- Jiang, Y.J. , Li, S.Z. , Li, R.P. , Zhang, J. , Liu, Y.H. , Lv, L.F. , et al. (2017) Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol Biochem 109: 145–155. [Google Scholar]
- Jin, Q. , Yuan, Z. , Xu, J. , Wang, Y. , Shen, Y. , Lu, W. , et al. (2002) Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res 30: 4432–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes, L. , Mikhail, J. , Bome‐Mannathoko, N. , Hadfield, S.J. , Harris, L.G. , El‐Bouri, K. , et al. (2010) Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase‐negative staphylococci and meticillin susceptibility testing directly from growth‐positive blood cultures by multiplex real‐time PCR. J Med Microbiol 59: 1456–1461. [DOI] [PubMed] [Google Scholar]
- Jünemann, S. , Kleinbölting, N. , Jaenicke, S. , Henke, C. , Hassa, J. , Nelkner, J. , et al. (2017) Bioinformatics for NGS‐based metagenomics and the application to biogas research. J Biotechnol 261: 10–23. [DOI] [PubMed] [Google Scholar]
- Kabir, S.M.L. , Chowdhury, N. , Asakura, M. , Shiramaru, S. , Kikuchi, K. , Hinenoya, A. , et al. (2019) Comparison of established PCR assays for accurate identification of Campylobacter jejuni and Campylobacter coli . Jpn J Infect Dis 72: 81–87. [DOI] [PubMed] [Google Scholar]
- Karimi, K. , Arzanlou, M. , and Pertot, I. (2019) Weeds as potential inoculum reservoir for Colletotrichum nymphaeae causing strawberry anthracnose in Iran and Rep‐PCR fingerprinting as useful marker to differentiate C. acutatum complex on strawberry. Front Microbiol 10: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. , Wang, R. , Li, B. , Liu, P. , Weng, Q. , and Chen, Q. (2018) Comparative evaluation of the LAMP Assay and PCR‐based assays for the rapid detection of Alternaria solani . Front Microbiol 9: 2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic, A. , and Basustaoglu, A.C. (2011) Double triplex real‐time PCR assay for simultaneous detection of Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus haemolyticus and determination of their methicillin resistance directly from positive blood culture bottles. Res Microbiol 162: 1060–1066. [DOI] [PubMed] [Google Scholar]
- Kim, H.‐J. , Ryu, J.‐O. , Song, J.‐Y. , and Kim, H.‐Y. (2017a) Multiplex polymerase chain reaction for identification of Shigellae and four Shigella species using novel genetic markers screened by comparative genomics. Foodborne Pathog Dis 14: 400–406. [DOI] [PubMed] [Google Scholar]
- Kim, J.S. , Kang, N.J. , Kwak, Y.S. , and Lee, C. (2017b) Investigation of genetic diversity of Fusarium oxysporum f. sp. fragariae using PCR‐RFLP. Plant Pathol J 33: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, H. , Oethinger, M. , Tuohy, M.J. , Hall, G.S. , and Bauer, T.W. (2009) Improving clinical significance of PCR: use of propidium monoazide to distinguish viable from dead Staphylococcus aureus and Staphylococcus epidermidis . J Orthop Res 27: 1243–1247. [DOI] [PubMed] [Google Scholar]
- Kou, L.P. , Gaskins, V.L. , Luo, Y.G. , and Jurick, W.M. 2nd (2014) First report of Alternaria tenuissima causing postharvest decay on apple fruit from cold storage in the United States. Plant Dis 98: 690. [DOI] [PubMed] [Google Scholar]
- Kumar, D. , Barad, S. , Sionov, E. , Keller, N.P. , and Prusky, D.B. (2017) Does the host contribute to modulation of mycotoxin production by fruit pathogens? Toxins 9: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda, M. , Ohta, T. , Uchiyama, I. , Baba, T. , Yuzawa, H. , Kobayashi, I. , et al. (2001) Whole genome sequencing of meticillin‐resistant Staphylococcus aureus . Lancet 357: 1225–1240. [DOI] [PubMed] [Google Scholar]
- Lamas, A. , Miranda, J.M. , Regal, P. , Vazquez, B. , Franco, C.M. , and Cepeda, A. (2018) A comprehensive review of non‐enterica subspecies of Salmonella enterica . Microbiol Res 206: 60–73. [DOI] [PubMed] [Google Scholar]
- Laothanachareon, T. , Tamayo‐Ramos, J.A. , Nijsse, B. , and Schaap, P.J. (2018) Forward genetics by genome sequencing uncovers the central role of the Aspergillus niger goxB locus in hydrogen peroxide induced glucose oxidase expression. Front Microbiol 9: 2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. , Iwata, T. , Shimizu, M. , Taniguchi, T. , Nakadai, A. , Hirota, Y. , et al. (2009) A novel multiplex PCR assay for Salmonella subspecies identification. J Appl Microbiol 107: 805–811. [DOI] [PubMed] [Google Scholar]
- Leff, J.W. , and Fierer, N. (2013) Bacterial communities associated with the surfaces of fresh fruits and vegetables. Plos One 8: e59310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, R.E. (2009) Molecular methods for detecting and discriminating Shigella associated with foods and human clinical infections a review. Food Biotechnol 23: 214–228. [Google Scholar]
- Li, S.S. , Cheng, C. , Li, Z. , Chen, J.Y. , Yan, B. , Han, B.Z. , et al. (2010) Yeast species associated with wine grapes in China. Int J Food Microbiol 138: 85–90. [DOI] [PubMed] [Google Scholar]
- Li, H.N. , Jiang, J.J. , Hong, N. , Wang, G.P. , and Xu, W.X. (2013) First report of Colletotrichum fructicola causing bitter rot of pear (Pyrus bretschneideri) in China. Plant Dis 97: 1000. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Mao, L. , Yan, D. , Ma, T. , Shen, J. , Guo, M. , et al. (2014) Quantification of Fusarium oxysporum in fumigated soils by a newly developed real‐time PCR assay to assess the efficacy of fumigants for Fusarium wilt disease in strawberry plants. Pest Manag Sci 70: 1669–1675. [DOI] [PubMed] [Google Scholar]
- Li, B. , Zong, Y. , Du, Z. , Chen, Y. , Zhang, Z. , Qin, G. , et al. (2015) Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol Plant Microbe Interact 28: 635–647. [DOI] [PubMed] [Google Scholar]
- Lichtemberg, P.S.F. , Luo, Y. , Doussoulin, H. , and Michailides, T.J. (2018) Using Allele‐specific PCR for detecting multiple amino acid substitutions associated with SDHI resistance in Alternaria alternata causing Alternaria late blight in pistachio. Lett Appl Microbiol 67: 506–512. [DOI] [PubMed] [Google Scholar]
- Lin, W.S. , Cheng, C.M. , and Van, K.T. (2010) A quantitative PCR assay for rapid detection of Shigella species in fresh produce. J Food Protect 73: 221–233. [DOI] [PubMed] [Google Scholar]
- Lindsey, R.L. , Fedorka‐Cray, P.J. , Abley, M. , Turpin, J.B. , and Meinersmann, R.J. (2015) Evaluating the occurrence of Escherichia albertii in chicken carcass rinses by PCR, Vitek analysis, and sequencing of the rpoB gene. Appl Environ Microbiol 81: 1727–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey, R.L. , Garcia‐Toledo, L. , Fasulo, D. , Gladney, L.M. , and Strockbine, N. (2017) Multiplex polymerase chain reaction for identification of Escherichia coli, Escherichia albertii and Escherichia fergusonii . J Microbiol Methods 140: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Lawrence, M.L. , Ainsworth, A.J. , and Austin, F.W. (2004) Species‐specific PCR determination of Listeria seeligeri. Res Microbiol 155: 741–746. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Li, Y. , Li, S. , Hu, N. , He, Y. , Pong, R. , et al. (2012) Comparison of next‐generation sequencing systems. J Biomed Biotechnol 2012: 251364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Wang, W. , Zhou, Y. , Yao, S. , Deng, L. , and Zeng, K. (2017) Isolation, identification and in vitro screening of Chongqing orangery yeasts for the biocontrol of Penicillium digitatum on citrus fruit. Biol Control 110: 18–24. [Google Scholar]
- Lleixa, J. , Kioroglou, D. , Mas, A. , and Portillo, M.D. (2018) Microbiome dynamics during spontaneous fermentations of sound grapes in comparison with sour rot and Botrytis infected grapes. Int J Food Microbiol 281: 36–46. [DOI] [PubMed] [Google Scholar]
- Llorens, A. , Hinojo, M.J. , Mateo, R. , Gonzalez‐Jaen, M.T. , Valle‐Algarra, F.M. , Logrieco, A. , et al. (2006) Characterization of Fusarium spp. isolates by PCR‐RFLP analysis of the intergenic spacer region of the rRNA gene (rDNA). Int J Food Microbiol 106: 297–306. [DOI] [PubMed] [Google Scholar]
- Lopes, U.P. , Zambolim, L. , Duarte, H.S.S. , Cabral, P.G.C. , Pereira, O.L. , Lopes, U.N. , et al. (2010) First report of leaf blight on Rubus brasiliensis caused by Colletotrichum acutatum in Brazil. Plant Dis 94: 1378. [DOI] [PubMed] [Google Scholar]
- Lopez‐Velasco, G. , Welbaum, G.E. , Boyer, R.R. , Mane, S.P. , and Ponder, M.A. (2011) Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage described using pyrosequencing of 16S rRNA amplicons. J Appl Microbiol 110: 1203–1214. [DOI] [PubMed] [Google Scholar]
- Lorenzini, M. , and Zapparoli, G. (2014) Characterization and pathogenicity of Alternaria spp. strains associated with grape bunch rot during post‐harvest withering. Int J Food Microbiol 186: 1–5. [DOI] [PubMed] [Google Scholar]
- Lu, H. , Giordano, F. , and Ning, Z. (2016) Oxford nanopore MinION sequencing and genome assembly. Genomics Proteomics Bioinformatics 14: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Y. , Gao, W. , Doster, M. , and Michailides, T.J. (2009) Quantification of conidial density of Aspergillus flavus and A. parasiticus in soil from almond orchards using real‐time PCR. J Appl Microbiol 106: 1649–1660. [DOI] [PubMed] [Google Scholar]
- Lüth, S. , Kleta, S. , and Al Dahouk, S. (2018) Whole genome sequencing as a typing tool for foodborne pathogens like Listeria monocytogenes–the way towards global harmonisation and data exchange. Trends Food Sci Technol 73: 67–75. [Google Scholar]
- Ma, L.J. , van der Does, H.C. , Borkovich, K.A. , Coleman, J.J. , Daboussi, M.J. , Di Pietro, A. , et al. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464: 67–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheux, A.F. , Boudreau, D.K. , Bergeron, M.G. , and Rodriguez, M.J. (2014) Characterization of Escherichia fergusonii and Escherichia albertii isolated from water. J Appl Microbiol 117: 97–609. [DOI] [PubMed] [Google Scholar]
- Mahmoud, M.A. (2015) Detection of Aspergillus flavus in stored peanuts using real‐time PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. flavus isolates. Foodborne Pathog Dis 12: 289–296. [DOI] [PubMed] [Google Scholar]
- Maifreni, M. , Frigo, F. , Bartolomeoli, I. , Innocente, N. , Biasutti, M. , and Marino, M. (2013) Identification of the Enterobacteriaceae in Montasio cheese and assessment of their amino acid decarboxylase activity. J Dairy Res 80: 122–127. [DOI] [PubMed] [Google Scholar]
- Marcet‐Houben, M. , Ballester, A.R. , Fuente, B.D.L. , Harries, E. , Marcos, J.F. , González‐Candelas, L. , et al. (2012) Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. Bmc Genomics 13: 646–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau, F. , Picard, F.J. , Lansac, N. , Menard, C. , Roy, P.H. , Ouellette, M. , et al. (2000) Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis . Antimicrob Agents Chemother 44: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam, A.M. , and Gilbert, W. (1977) A new method for sequencing DNA. Proc Natl Acad Sci USA 74: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland, M. , Sanderson, K.E. , Spieth, J. , Clifton, S.W. , Latreille, P. , Courtney, L. , et al. (2001) Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413: 852–856. [DOI] [PubMed] [Google Scholar]
- Medina, A. , Mateo, R. , Lopez‐Ocana, L. , Valle‐Algarra, F.M. , and Jimenez, M. (2005) Study of Spanish grape mycobiota and ochratoxin A production by Isolates of Aspergillus tubingensis and other members of Aspergillus section Nigri. Appl Environ Microbiol 71: 4696–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes Silva, D. , and Domingues, L. (2015) On the track for an efficient detection of Escherichia coli in water: a review on PCR‐based methods. Ecotoxicol Environ Saf 113: 400–411. [DOI] [PubMed] [Google Scholar]
- Mezzasalma, V. , Sandionigi, A. , Bruni, I. , Bruno, A. , Lovicu, G. , Casiraghi, M. , et al. (2017) Grape microbiome as a reliable and persistent signature of field origin and environmental conditions in Cannonau wine production. Plos One 12: e0184615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzasalma, V. , Sandionigi, A. , Guzzetti, L. , Galimberti, A. , Grando, M.S. , Tardaguila, J. , et al. (2018) Geographical and cultivar features differentiate grape microbiota in Northern Italy and Spain Vineyards. Front Microbiol 9: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheyev, A.S. , and Tin, M.M. (2014) A first look at the Oxford Nanopore MinION sequencer. Mol Ecol Resour 14: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Milanovic, V. , Comitini, F. , and Ciani, M. (2013) Grape berry yeast communities: influence of fungicide treatments. Int J Food Microbiol 161: 240–246. [DOI] [PubMed] [Google Scholar]
- Mirhendi, H. , Zarei, F. , Motamedi, M. , and Nouripour‐Sisakht, S. (2016) Aspergillus tubingensis and Aspergillus niger as the dominant black Aspergillus, use of simple PCR‐RFLP for preliminary differentiation. J Mycol Med 26: 9–16. [DOI] [PubMed] [Google Scholar]
- Mohmed, M. , Abd‐Elsalam, K. , Mohamed, Y. , and Ali, B. (2010) First morphomolecular identification of Penicillium griseofulvum and Penicillium aurantiogriseum toxicogenic isolates associated with blue mold on apple. Foodborne Pathog Dis 7: 857–861. [DOI] [PubMed] [Google Scholar]
- Moncrief, I. , Garzon, C. , Marek, S. , Stack, J. , Gamliel, A. , Garrido, P. , et al. (2016) Development of simple sequence repeat (SSR) markers for discrimination among isolates of Fusarium proliferatum . J Microbiol Methods 126: 12–17. [DOI] [PubMed] [Google Scholar]
- Moore, G.G. , Rakhi, S. , Horn, B.W. , and Ignazio, C. (2010) Recombination and lineage‐specific gene loss in the aflatoxin gene cluster of Aspergillus flavus. Mol Ecol 18: 4870–4887. [DOI] [PubMed] [Google Scholar]
- Morey, M. , Fernández‐Marmiesse, A. , Castiñeiras, D. , Fraga, J.M. , Couce, M.L. , and Cocho, J.A. (2013) A glimpse into past, present, and future DNA sequencing. Mol Genet Metabol 110: 3–24. [DOI] [PubMed] [Google Scholar]
- Morrison‐Whittle, P. , Lee, S.A. , and Goddard, M.R. (2017) Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agric Ecosyst Environ 246: 306–313. [Google Scholar]
- Mulè, G. , Susca, A. , Stea, G. , and Moretti, A. (2004) A species‐specific pcr assay based on the calmodulin partial gene for identification offusarium verticillioides, f. proliferatum and f. subglutinans. Eur J Plant Pathol 110: 495–502. [Google Scholar]
- Munoz, G. , Campos, F. , Salgado, D. , Galdames, R. , Gilchrist, L. , Chahin, G. , et al. (2016) Molecular identification of Botrytis cinerea, Botrytis paeoniae and Botrytis pseudocinerea associated with gray mould disease in peonies (Paeonia lactiflora Pall.) in Southern Chile. Rev Iberoam Micol 33: 43–47. [DOI] [PubMed] [Google Scholar]
- Muraosa, Y. , Schreiber, A.Z. , Trabasso, P. , Matsuzawa, T. , Taguchi, H. , Moretti, M.L. , et al. (2014) Development of cycling probe‐based real‐time PCR system to detect Fusarium species and Fusarium solani species complex (FSSC). Int J Med Microbiol 304: 505–511. [DOI] [PubMed] [Google Scholar]
- Mylroie, J.E. , Ozkan, S. , Shivaji, R. , Windham, G.L. , Alpe, M.N. , and Williams, W.P. (2016) Identification and quantification of a toxigenic and non‐toxigenic Aspergillus flavus strain in contaminated maize using quantitative real‐time PCR. Toxins 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo‐Ortiz, M.A. , Rodriguez‐Pires, S. , Torres, R. , De Cal, A. , Usall, J. , and Gabaldon, T. (2018) Genome sequence of the brown rot fungal pathogen Monilinia laxa . Genome Announc 6: e00214‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri, T. , Hedayati, M.T. , Abastabar, M. , Pasqualotto, A.C. , Armaki, M.T. , Hoseinnejad, A. , et al. (2015) PCR‐RFLP on β‐Tubulin gene for rapid identification of the most clinically important species of Aspergillus. J Microbiol Methods 117: 144–147. [DOI] [PubMed] [Google Scholar]
- Ng, L.K. , Kingombe, C.I. , Yan, W. , Taylor, D.E. , Hiratsuka, K. , Malik, N. , et al. (1997) Specific detection and confirmation of Campylobacter jejuni by DNA hybridization and PCR. Appl Environ Microbiol 63: 4558–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, H.D. , Lewis, C.T. , Levesque, C.A. , and Grafenhan, T. (2016) Draft genome sequence of Alternaria alternata ATCC 34957. Genome Announc 4: e01554‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, J. (2017) Occurrence, control and determination of patulin contamination in fruits and fruit products. Sci Agric Sin 50: 3591–3607. [Google Scholar]
- Niehaus, E.M. , Munsterkotter, M. , Proctor, R.H. , Brown, D.W. , Sharon, A. , Idan, Y. , et al. (2016) Comparative “Omics” of the Fusarium fujikuroi species complex highlights differences in genetic potential and metabolite synthesis. Genome Biol Evol 8: 3574–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, L.K. , Jensen, J.D. , Rodriguez, A. , Jorgensen, L.N. , and Justesen, A.F. (2012) TRI12 based quantitative real‐time PCR assays reveal the distribution of trichothecene genotypes of F. graminearum and F. culmorum isolates in Danish small grain cereals. Int J Food Microbiol 157: 384–392. [DOI] [PubMed] [Google Scholar]
- Nierman, W.C. , Yu, J.J. , Fedorova‐Abrams, N.D. , Losada, L. , Cleveland, T.E. , Bhatnagar, D. , et al. (2015) Genome sequence of Aspergillus flavus NRRL 3357, a strain that causes aflatoxin contamination of food and feed. Genome Announc 3: e00168‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormohamed, A. , and Fakhr, M.K. (2013) Arsenic resistance and prevalence of arsenic resistance genes in Campylobacter jejuni and Campylobacter coli isolated from retail meats. Int J Environ Res Public Health 10: 3453–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, L. , and Glynn, B. (2010) Recent advances in the development of nucleic acid diagnostics. Expert Rev Med Devices 7: 529–539. [DOI] [PubMed] [Google Scholar]
- Oh, J.Y. , Kwon, Y.K. , Wei, B. , Jang, H.K. , Lim, S.K. , Kim, C.H. , et al. (2017) Epidemiological relationships of Campylobacter jejuni strains isolated from humans and chickens in South Korea. J Microbiol 55: 13–20. [DOI] [PubMed] [Google Scholar]
- Ojha, S.C. , Yean, C.Y. , Ismail, A. , and Singh, K.K.B. (2013) A Pentaplex PCR assay for the detection and differentiation of Shigella species. Biomed Res Int 9: 412370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaimat, A.N. , and Holley, R.A. (2012) Factors influencing the microbial safety of fresh produce: a review. Food Microbiol 32: 1–19. [DOI] [PubMed] [Google Scholar]
- Omori, A.M. , Ono, E.Y.S. , Bordini, J.G. , Hirozawa, M.T. , Fungaro, M.H.P. , and Ono, M.A. (2018) Detection of Fusarium verticillioides by PCR‐ELISA based on FUM21 gene. Food Microbiol 73: 160–167. [DOI] [PubMed] [Google Scholar]
- Omwenga, I. , Aboge, G.O. , Mitema, E.S. , Obiero, G. , Ngaywa, C. , Ngwili, N. , et al. (2019) Staphylococcus aureus enterotoxin genes detected in milk from various livestock species in northern pastoral region of Kenya. Food Control 103: 126–132. [Google Scholar]
- Ooka, T. , Terajima, J. , Kusumoto, M. , Iguchi, A. , Kurokawa, K. , Ogura, Y. , et al. (2009) Development of a multiplex PCR‐based rapid typing method for enterohemorrhagic Escherichia coli O157 strains. J Clin Microbiol 47: 2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, S.F. , Del Pilar Bustos Lopez, M. , Nari, L. , Boonham, N. , Gullino, M.L. , and Spadaro, D. (2019) Rapid detection of Monilinia fructicola and Monilinia laxa on Peach and Nectarine using loop‐mediated isothermal amplification. Plant Dis 103: 2305–2314. [DOI] [PubMed] [Google Scholar]
- Ortiz, C.S. , Bell, A.A. , Magill, C.W. , and Liu, J. (2017) Specific PCR detection of Fusarium oxysporum f. sp. vasinfectum California Race 4 based on a unique Tfo1 insertion event in the PHO gene. Plant Dis 101: 34–44. [DOI] [PubMed] [Google Scholar]
- Osek, J. (2001) Multiplex polymerase chain reaction assay for identification of enterotoxigenic Escherichia coli strains. J Vet Diagn Investig 13: 308–311. [DOI] [PubMed] [Google Scholar]
- Oshikata, C. , Tsurikisawa, N. , Saito, A. , Watanabe, M. , Kamata, Y. , Tanaka, M. , et al. (2013) Fatal pneumonia caused by Penicillium digitatum: a case report. BMC Pulmonary Med 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani Bojd, M. , Kamaladini, H. , Haddadi, F. , and Vaseghi, A. (2017) Thiolated AuNP probes and multiplex PCR for molecular detection of Staphylococcus epidermidis . Mol Cell Probes 34: 30–36. [DOI] [PubMed] [Google Scholar]
- Ostry, V. , Malir, F. , Cumova, M. , Kyrova, V. , Toman, J. , Grosse, Y. , et al. (2018) Investigation of patulin and citrinin in grape must and wine from grapes naturally contaminated by strains of Penicillium expansum. Food Chem Toxicol 118: 805–811. [DOI] [PubMed] [Google Scholar]
- Ottesen, A.R. , Pena, A.G. , White, J.R. , Pettengill, J.B. , Li, C. , Allard, S. , et al. (2013) Baseline survey of the anatomical microbial ecology of an important food plant: Solanum lycopersicum (tomato). BMC Microbiol 13: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard, D. , Dubois, V. , Duran, R. , Nathier, F. , Guittet, C. , Caumette, P. , et al. (2003) Rapid identification of Listeria species by using restriction fragment length polymorphism of PCR‐amplified 23S rRNA gene fragments. Appl Environ Microbiol 69: 6386–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva‐Santos, W. , Barros, E.M. , Sousa, V.S. , Laport, M.S. , and Giambiagi‐deMarval, M. (2016) Identification of coagulase‐negative Staphylococcus saprophyticus by polymerase chain reaction based on the heat‐shock repressor encoding hrcA gene. Diagn Microbiol Infect Dis 86: 253–256. [DOI] [PubMed] [Google Scholar]
- Palumbo, J.D. , and O'Keeffe, T.L. (2015) Detection and discrimination of four Aspergillus section Nigri species by PCR. Lett Appl Microbiol 60: 188–195. [DOI] [PubMed] [Google Scholar]
- Papavasileiou, A. , Madesis, P.B. , and Karaoglanidis, G.S. (2016) Identification and differentiation of Monilinia species causing brown rot of pome and stone fruit using high‐resolution melting (HRM) analysis. Phytopathology 106: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Parkhill, J. , Wren, B.W. , Mungall, K. , Ketley, J.M. , Churcher, C. , Basham, D. , et al. (2000) The genome sequence of the food‐borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403: 665–668. [DOI] [PubMed] [Google Scholar]
- Parkhill, J. , Dougan, G. , James, K.D. , Thomson, N.R. , Pickard, D. , Wain, J. , et al. (2001) Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413: 848–852. [DOI] [PubMed] [Google Scholar]
- Pavlova, M.R. , Dobreva, E.G. , Ivanova, K.I. , Asseva, G.D. , Ivanov, I.N. , Petrov, P.K. , et al. (2016) Multiplex PCR assay for identifi cation and differentiation of Campylobacter jejuni and Campylobacter coli isolates. Folia Med (Plovdiv) 58: 95–100. [DOI] [PubMed] [Google Scholar]
- Peterson, G. , Gerdes, B. , Berges, J. , Nagaraja, T.G. , Frye, J.G. , Boyle, D.S. , et al. (2010) Development of microarray and multiplex polymerase chain reaction assays for identification of serovars and virulence genes in Salmonella enterica of human or animal origin. J Vet Diagn Investig 22: 559–569. [DOI] [PubMed] [Google Scholar]
- Phumthanakorn, N. , Chanchaithong, P. , and Prapasarakul, N. (2017) Development of a set of multiplex PCRs for detection of genes encoding cell wall‐associated proteins in Staphylococcus pseudintermedius isolates from dogs, humans and the environment. J Microbiol Methods 142: 90–95. [DOI] [PubMed] [Google Scholar]
- Piao, H. , Hawley, E. , Kopf, S. , DeScenzo, R. , Sealock, S. , Henick‐Kling, T. , et al. (2015) Insights into the bacterial community and its temporal succession during the fermentation of wine grapes. Front Microbiol 6: 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileggi, S.A. , Vieira de Oliveira, S.F. , Andrade, C.W. , Vicente, V.A. , Dalzoto Pdo, R. , Kniphoff da Cruz, G. , et al. (2009) Molecular and morphological markers for rapid distinction between 2 Colletotrichum species. Can J Microbiol 55: 1076–1088. [DOI] [PubMed] [Google Scholar]
- Pinheiro, L. , Brito, C.I. , de Oliveira, A. , Martins, P.Y. , Pereira, V.C. , and da Cunha Mde, L. (2015) Staphylococcus epidermidis and Staphylococcus haemolyticus: molecular detection of cytotoxin and enterotoxin genes. Toxins 7: 3688–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, C. , Pinho, D. , Sousa, S. , Pinheiro, M. , Egas, C. , and Gomes, A.C. (2014) Unravelling the diversity of grapevine microbiome. PLoS ONE 9: e85622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piombo, E. , Sela, N. , Wisniewski, M. , Hoffmann, M. , Gullino, M.L. , Allard, M.W. , et al. (2018) Genome sequence, assembly and characterization of two Metschnikowia fructicola Strains used as biocontrol agents of postharvest diseases. Front Microbiol 9: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi, G. , Aiello, D. , Guarnaccia, V. , Vitale, A. , Perrone, G. , and Stea, G. (2011) First report of damping‐off on Strawberry Tree caused by Colletotrichum acutatum and C. simmondsii in Italy. Plant Dis 95: 1588. [DOI] [PubMed] [Google Scholar]
- Portillo, M.D. , and Mas, A. (2016) Analysis of microbial diversity and dynamics during wine fermentation of Grenache grape variety by high‐throughput barcoding sequencing. LWT‐Food Sci Technol 72: 317–321. [Google Scholar]
- Portillo, M.D.C. , Franquès, J. , Araque, I. , Reguant, C. , and Bordons, A. (2016) Bacterial diversity of Grenache and Carignan grape surface from different vineyards at Priorat wine region (Catalonia, Spain). Int J Food Microbiol 219: 56–63. [DOI] [PubMed] [Google Scholar]
- Postollec, F. , Falentin, H. , Pavan, S. , Combrisson, J. , and Sohier, D. (2011) Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol 28: 848–861. [DOI] [PubMed] [Google Scholar]
- Proctor, R.H. , and Vaughan, M.M. (2017) Targeting Fumonisin biosynthetic genes. Methods Mol Biol 1542: 201–214. [DOI] [PubMed] [Google Scholar]
- Puel, O. , Galtier, P. , and Oswald, I.P. (2010) Biosynthesis and toxicological effects of Patulin. Toxins 2: 613–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese, M. , Ferrocino, I. , Gullino, M.L. , and Garibaldi, A. (2013) Detection of Fusarium oxysporum f.sp. basilici in substrates and roots by PCR. Commun Agric Appl Biol Sci 78: 621–624. [PubMed] [Google Scholar]
- Purahong, W. , Orru, L. , Donati, I. , Perpetuini, G. , Cellini, A. , Lamontanara, A. , et al. (2018) Plant microbiome and its link to plant health: host species, organs and Pseudomonas syringae pv. actinidiae infection shaping bacterial phyllosphere communities of Kiwifruit plants. Front Plant Science 9: 1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkarev, D. , Neff, N.F. , and Quake, S.R. (2009) Single‐molecule sequencing of an individual human genome. Nat Biotechnol 27: 847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhika, M. , Saugata, M. , Murali, H.S. , and Batra, H.V. (2014) A novel multiplex PCR for the simultaneous detection of Salmonella enterica and Shigella species. Brazil J Microbiol 45: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdeen, S. , and Rampersad, S.N. (2013) Intraspecific differentiation of Colletotrichum gloeosporioides sensu lato based on in silico multilocus PCR‐RFLP fingerprinting. Mol Biotechnol 53: 170–181. [DOI] [PubMed] [Google Scholar]
- Rastogi, G. , Sbodio, A. , Tech, J.J. , Suslow, T.V. , Coaker, G.L. , and Leveau, J.H.J. (2012) Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field‐grown lettuce. ISME J 6: 1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich, J.D. , Alexander, T.W. , and Chatterton, S. (2016) A multiplex PCR assay for the detection and quantification of Sclerotinia sclerotiorum and Botrytis cinerea. Lett Appl Microbiol 62: 379–385. [DOI] [PubMed] [Google Scholar]
- Reuter, Jason A. , Spacek, D.V. , and Snyder, Michael P. (2015) High‐throughput sequencing technologies. Mol Cell 58: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende, A.C.B. , Crucello, J. , Moreira, R.C. , Silva, B.S. , and Sant'Ana, A.S. (2016) Incidence and growth of Salmonella enterica on the peel and pulp of avocado (Persea americana) and custard apple (Annona squamosa). Int J Food Microbiol 235: 10–16. [DOI] [PubMed] [Google Scholar]
- Rharmitt, S. , Hafidi, M. , Hajjaj, H. , Scordino, F. , Giosa, D. , Giuffre, L. , et al. (2016) Molecular characterization of patulin producing and non‐producing Penicillium species in apples from Morocco. Int J Food Microbiol 217: 137–140. [DOI] [PubMed] [Google Scholar]
- Ribic, U. , Klancnik, A. , and Jersek, B. (2017) Characterization of Staphylococcus epidermidis strains isolated from industrial cleanrooms under regular routine disinfection. J Appl Microbiol 122: 1186–1196. [DOI] [PubMed] [Google Scholar]
- Rigotti, S. , Gindro, K. , Richter, H. , and Viret, O. (2002) Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: Fr. in strawberry (Fragariaxananassa Duch.) using PCR. FEMS Microbiol Lett 209: 169–174. [DOI] [PubMed] [Google Scholar]
- Rodgers, J.D. , Simpkin, E. , Lee, R. , Clifton‐Hadley, F.A. , and Vidal, A.B. (2017) Sensitivity of direct culture, enrichment and PCR for detection of Campylobacter jejuni and C. coli in Broiler Flocks at Slaughter. Zoonoses Public Health 64: 262–271. [DOI] [PubMed] [Google Scholar]
- Rogers, S. , Girolami, M. , Kolch, W. , Waters, K.M. , Liu, T. , Thrall, B. , et al. (2008) Investigating the correspondence between transcriptomic and proteomic expression profiles using coupled cluster models. Bioinformatics 24: 2894–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl, J.W. , Morris, C.R. , Emberger, J. , Fraser, C.M. , Ochieng, J.B. , Juma, J. , et al. (2015) Defining the phylogenomics of Shigella species: a pathway to diagnostics. J Clin Microbiol 53: 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sails, A.D. , Fox, A.J. , Bolton, F.J. , Wareing, D.R. , Greenway, D.L. , and Borrow, R. (2001) Development of a PCR ELISA assay for the identification of Campylobacter jejuni and Campylobacter coli . Mol Cell Probes 15: 291–300. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Vargas, F.M. , Abu‐El‐Haija, M.A. , and Gomez‐Duarte, O.G. (2011) Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis 9: 263–277. [DOI] [PubMed] [Google Scholar]
- Sanger, F. , and Coulson, A.R. (1975) A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol 94: 441–448. [DOI] [PubMed] [Google Scholar]
- Sanger, F. , Donelson, J.E. , Coulson, A.E. , Kössel, H. , and Fischer, D. (1974). Determination of a nucleotide sequence in bacteriophage f1 DNA by primed synthesis with DNA polymerase. J Mol Biol 90: 315, IN333,327–326, IN341,333. [DOI] [PubMed] [Google Scholar]
- Sanger, F. , Air, G.M. , Barrell, B.G. , Brown, N.L. , Coulson, A.R. , Fiddes, J.C. , et al. (1977) Nucleotide sequence of bacteriophage φX174 DNA. Nature 265: 687–695. [DOI] [PubMed] [Google Scholar]
- Sanger, F. , Coulson, A.R. , Hong, G.F. , Hill, D.F. , and Petersen, G.B. (1983) Nucleotide sequence of bacteriophage λ DNA. J Mol Biol 162: 729–773. [DOI] [PubMed] [Google Scholar]
- Schena, L. , Ippolito, A. , Zahavi, T. , Cohen, L. , and Droby, S. (2000) Molecular approaches to assist the screening and monitoring of postharvest biocontrol yeasts. Eur J Plant Pathol 106: 681–691. [Google Scholar]
- Shade, A. , McManus, P.S. , and Handelsman, J. (2013) Unexpected diversity during community succession in the apple flower microbiome. Mbio 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, G. , Maymon, M. , and Freeman, S. (2017) Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci Rep 7: 15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, T.R. , Devanna, B.N. , Kiran, K. , Singh, P.K. , Arora, K. , Jain, P. , et al. (2018) Status and prospects of next‐generation sequencing technologies in crop plants. Curr Issues Mol Biol 27: 1–36. [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Nie, J. , Dong, Y. , Kuang, L. , Li, Y. , and Zhang, J. (2018a) Compositional shifts in the surface fungal communities of apple fruits during cold storage. Postharvest Biol Technol 144: 55–62. [Google Scholar]
- Shen, Y. , Nie, J. , Li, Z. , Li, H. , Wu, Y. , Dong, Y. , et al. (2018b) Differentiated surface fungal communities at point of harvest on apple fruits from rural and peri‐urban orchards. Sci Rep 8: 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z. , Bai, S. , Tian, L. , Jiang, H. , and Zhang, J. (2011) Molecular detection of Penicillium griseofulvum as the coastal pollution indicator. Curr Microbiol 62: 396–401. [DOI] [PubMed] [Google Scholar]
- Sierra‐Arguello, Y.M. , Quedi Furian, T. , Perdoncini, G. , Moraes, H.L.S. , Salle, C.T.P. , Rodrigues, L.B. , et al. (2018) Fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli from poultry and human samples assessed by PCR‐restriction fragment length polymorphism assay. PLoS One 13: e0199974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, L.L. , Pestana, K.N. , Ferreira, C.F. , and Olveira, S.A.S. (2018) Differentiation of phylogenetic lineages within the ‘Colletotrichum gloeosporioides species complex’ associated with cassava anthracnose disease by PCR‐RFLP. Trop Plant Pathol 43: 194–201. [Google Scholar]
- Simmons, K. , Rempel, H. , Block, G. , Forgetta, V. , Vaillancourt, R. Jr , Malouin, F. , et al. (2014) Duplex PCR methods for the molecular detection of Escherichia fergusonii isolates from broiler chickens. Appl Environ Microbiol 80: 1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , and Kapoor, R. (2018) Quick and accurate detection of Fusarium oxysporum f. sp. carthami in host tissue and soil using conventional and real‐time PCR assay. World J Microbiol Biotechnol 34: 175. [DOI] [PubMed] [Google Scholar]
- Singh, R. , Kumar, S. , Kashyap, P.L. , Srivastava, A.K. , Mishra, S. , and Sharma, A.K. (2014) Identification and characterization of microsatellite from Alternaria brassicicola to assess cross‐species transferability and utility as a diagnostic marker. Mol Biotechnol 56: 1049–1059. [DOI] [PubMed] [Google Scholar]
- Siroli, L. , Patrignani, F. , Serrazanetti, D.I. , Gardini, F. , and Lanciotti, R. (2015) Innovative strategies based on the use of bio‐control agents to improve the safety, shelf‐life and quality of minimally processed fruits and vegetables. Trends Food Sci Technol 46: 302–310. [Google Scholar]
- Siroli, L. , Patrignani, F. , Serrazanetti, D.I. , Vernocchi, P. , Del Chierico, F. , Russo, A. , et al. (2017) Effect of thyme essential oil and Lactococcus lactis CBM21 on the microbiota composition and quality of minimally processed lamb's lettuce. Food Microbiol 68: 61–70. [DOI] [PubMed] [Google Scholar]
- Skow, A. , Mangold, K.A. , Tajuddin, M. , Huntington, A. , Fritz, B. , Thomson, R.B. Jr , et al. (2005) Species‐level identification of staphylococcal isolates by real‐time PCR and melt curve analysis. J Clin Microbiol 43: 2876–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler‐Garcia, A.A. , De Jesus, A.J. , Taylor, K. , and Brown, E.W. (2014) Differentiation of Salmonella strains from the SARA, SARB and SARC reference collections by using three genes PCR‐RFLP and the 2100 Agilent Bioanalyzer. Front Microbiol 5: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman, S. , Li, X.Z. , Shao, S. , Behar, M. , Svircev, A.M. , Tsao, R. , et al. (2015) Potential mycotoxin contamination risks of apple products associated with fungal flora of apple core. Food Control 47: 585–591. [Google Scholar]
- Somashekar, D. , Rati, E.R. , and Chandrashekar, A. (2004) PCR‐restriction fragment length analysis of aflR gene for differentiation and detection of Aspergillus flavus and Aspergillus parasiticus in maize. Int J Food Microbiol 93: 101–107. [DOI] [PubMed] [Google Scholar]
- Somma, S. , Amatulli, M.T. , Masiello, M. , Moretti, A. , and Logrieco, A.F. (2019) Alternaria species associated to wheat black point identified through a multilocus sequence approach. Int J Food Microbiol 293: 34–43. [DOI] [PubMed] [Google Scholar]
- Song, J. , Gu, W. , Lin, C. , Zhang, X. , Gan, Z. , Xie, L. , et al. (2018) Molecular identification and safety evaluation of Penicillium citrinum YL‐1 from fish sauce based on fungal genomic sequencing. Shipin Kexue/Food Sci 39: 305–311. [Google Scholar]
- Song, M. , Li, Q. , He, Y. , Lan, L. , Feng, Z. , Fan, Y. , et al. (2019) A comprehensive multilocus sequence typing scheme for identification and genotyping of Staphylococcus strains. Foodborne Pathog Dis 16: 331–338. [DOI] [PubMed] [Google Scholar]
- Steenkamp, E.T. , Wingfield, B.D. , Coutinho, T.A. , Wingfield, M.J. , and Marasas, W.F. (1999) Differentiation of Fusarium subglutinans f. sp. pini by histone gene sequence data. Appl Environ Microbiol 65: 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp, E.T. , Wingfield, B.D. , Coutinho, T.A. , Zeller, K.A. , Wingfield, M.J. , Marasas, W.F. , et al. (2000) PCR‐based identification of MAT‐1 and MAT‐2 in the Gibberella fujikuroi species complex. Appl Environ Microbiol 66: 4378–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini, I. , Albanese, D. , Cavazza, A. , Franciosi, E. , De Filippo, C. , Donati, C. , et al. (2016) Dynamic changes in microbiota and mycobiota during spontaneous “Vino Santo Trentino” fermentation. Microb Biotechnol 9: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini, I. , Carlin, S. , Tocci, N. , Albanese, D. , Donati, C. , Franceschi, P. , et al. (2017) Core microbiota and metabolome of Vitis vinifera L. cv. Corvina grapes and musts. Front Microbiol 8: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, C. , and Murray, W. (2001) Pathogen evolution: how good bacteria go bad. Curr Biol 11: R53–R56. [DOI] [PubMed] [Google Scholar]
- Sternes, P.R. , Lee, D. , Kutyna, D.R. , and Borneman, A.R. (2017) A combined meta‐barcoding and shotgun metagenomic analysis of spontaneous wine fermentation. Gigascience 6: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez, M.B. , Walsh, K. , Boonham, N. , O'Neill, T. , Pearson, S. , and Barker, I. (2005) Development of real‐time PCR (TaqMan) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiol Biochem 43: 890–899. [DOI] [PubMed] [Google Scholar]
- Sun, D.‐L. , Jiang, X. , Wu, Q.L. , and Zhou, N.‐Y. (2013) Intragenomic heterogeneity of 16S rRNA genes causes overestimation of prokaryotic diversity. Appl Environ Microbiol 79: 5962–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagar, R. , Deore, S.N. , Deshpande, P.V. , Rizwan, A. , Sannejal, A.D. , Sundareshan, S. , et al. (2013) Differentiation of Staphylococcus aureus and Staphylococcus epidermidis by PCR for the fibrinogen binding protein gene. J Dairy Sci 96: 2857–2865. [DOI] [PubMed] [Google Scholar]
- Talhinhas, P. , Sreenivasaprasad, S. , Neves‐Martins, J. , and Oliveira, H. (2002) Genetic and morphological characterization of Colletotrichum acutatum causing Anthracnose of lupins. Phytopathology 92: 986–996. [DOI] [PubMed] [Google Scholar]
- Talhinhas, P. , Sreenivasaprasad, S. , Neves‐Martins, J. , and Oliveira, H. (2005) Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl Environ Microbiol 71: 2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous, J. , Atoui, A. , El Khoury, A. , Kantar, S. , Chdid, N. , Oswald, I.P. , et al. (2015) Development of a real‐time PCR assay for Penicillium expansum quantification and patulin estimation in apples. Food Microbiol 50: 28–37. [DOI] [PubMed] [Google Scholar]
- Tapia‐Tussell, R. , Quijano‐Ramayo, A. , Cortes‐Velazquez, A. , Lappe, P. , Larque‐Saavedra, A. , and Perez‐Brito, D. (2008) PCR‐based detection and characterization of the fungal pathogens Colletotrichum gloeosporioides and Colletotrichum capsici causing anthracnose in papaya (Carica papaya l.) in the Yucatan peninsula. Mol Biotechnol 40: 293–298. [DOI] [PubMed] [Google Scholar]
- Thompson, J.R. , and Latorre, B.A. (1999) Characterization of Botrytis cinerea from Table Grapes in Chile using RAPD‐PCR. Plant Dis 83: 1090–1094. [DOI] [PubMed] [Google Scholar]
- Tindall, B.J. , Grimont, P.A.D. , Garrity, G.M. , and Euzeby, J.P. (2005) Nomenclature and taxonomy of the genus Salmonella. Int J Syst Evol Microbiol 55: 521–524. [DOI] [PubMed] [Google Scholar]
- Toledo del Arbol, J. , Perez Pulido, R. , La Storia, A. , Grande Burgos, M.J. , Lucas, R. , Ercolini, D. , et al. (2016) Microbial diversity in pitted sweet cherries (Prunus avium L.) as affected by high‐hydrostatic pressure treatment. Food Res Int 89: 790–796. [DOI] [PubMed] [Google Scholar]
- Torres‐Cortes, G. , Bonneau, S. , Bouchez, O. , Genthon, C. , Briand, M. , Jacques, M.A. , et al. (2018) Functional microbial features driving community assembly during seed germination and emergence. Front Plant Sci 9: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tralamazza, S.M. , Braghini, R. , and Correa, B. (2016) Trichothecene genotypes of the Fusarium graminearum species complex isolated from Brazilian wheat grains by conventional and quantitative PCR. Front Microbiol 7: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchado, P. , Gil, M.I. , Suslow, T. , and Allende, A. (2018) Impact of chlorine dioxide disinfection of irrigation water on the epiphytic bacterial community of baby spinach and underlying soil. PLoS ONE 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryfinopoulou, P. , Kizis, D. , Nychas, G.J. , and Panagou, E.Z. (2015) Quantification of Aspergillus carbonarius in grapes using a real time PCR assay. Food Microbiol 51: 139–143. [DOI] [PubMed] [Google Scholar]
- Tuntevski, K. , Durney, B.C. , Snyder, A.K. , Lasala, P.R. , Nayak, A.P. , Green, B.J. , et al. (2013) Aspergillus collagen‐like genes (acl): identification, sequence polymorphism, and assessment for PCR‐based pathogen detection. Appl Environ Microbiol 79: 7882–7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyaguari‐Diaz, M.I. , Chan, M. , Chaban, B.L. , Croxen, M.A. , Finke, J.F. , Hill, J.E. , et al. (2016) A comprehensive method for amplicon‐based and metagenomic characterization of viruses, bacteria, and eukaryotes in freshwater samples. Microbiome 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Välimaa, A.‐L. , Tilsala‐Timisjärvi, A. , and Virtanen, E. (2015) Rapid detection and identification methods for Listeria monocytogenes in the food chain – a review. Food Control 55: 103–114. [Google Scholar]
- Vannuffel, P. , Heusterspreute, M. , Bouyer, M. , Vandercam, B. , Philippe, M. , and Gala, J.L. (1999) Molecular characterization of femA from Staphylococcus hominis and Staphylococcus saprophyticus, and femA‐based discrimination of staphylococcal species. Res Microbiol 150: 129–141. [DOI] [PubMed] [Google Scholar]
- Verstappen, K.M. , Huijbregts, L. , Spaninks, M. , Wagenaar, J.A. , Fluit, A.C. , and Duim, B. (2017) Development of a real‐time PCR for detection of Staphylococcus pseudintermedius using a novel automated comparison of whole‐genome sequences. PLoS One 12: e0183925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk, C. , Heide, R.V.D. , Vries, I.D. , Lee, T.V.D. , Schoen, C. , Corainville, C.D. , et al. (2004) Quantitative detection of Fusarium species in wheat using TaqMan. Eur J Plant Pathol 110: 481–494. [Google Scholar]
- Wang, C.L. , and Cheng, Y.H. (2017) Identification and trichothecene genotypes of Fusarium graminearum species complex from wheat in Taiwan. Bot Stud 58: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , García‐Fernández, D. , Mas, A. , and Esteve‐Zarzoso, B. (2015). Fungal diversity in grape must and wine fermentation assessed by massive sequencing, quantitative PCR and DGGE. Front Microbiol 6: 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F. , Chen, L. , Liu, S.J. , Li, F.Q. , Zhang, X. , Chen, H.P. , et al. (2018a) Studying safe storage time of orange peel (Citrus reticulata) using high‐throughput sequencing and conventional pure culture. Food Sci Nutr 6: 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.R. , Guo, L.Y. , Xiao, C.L. , and Zhu, X.Q. (2018b) Detection and identification of six Monilinia spp. causing brown rot using TaqMan Real‐Time PCR from pure cultures and infected apple fruit. Plant Dis 102: 1527–1533. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Zhao, J. , Ma, G. , Bao, S. , and Wu, X. (2019) Leaf blight of sunflower caused by Alternaria tenuissima and A. alternata in Beijing, China. Can J Plant Pathol 41: 372–378. [Google Scholar]
- Warren, B.R. , Parish, M.E. , and Schneider, K.R. (2006) Shigella as a foodborne pathogen and current methods for detection in food. Crit Rev Food Sci Nutr 46: 551–567. [DOI] [PubMed] [Google Scholar]
- Wassermann, B. , Rybakova, D. , Müller, C. , and Berg, G. (2017) Harnessing the microbiomes of Brassica vegetables for health issues. Sci Rep 7: 17649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann, P. , Sieber, C.M. , von Bargen, K.W. , Studt, L. , Niehaus, E.M. , Espino, J.J. , et al. (2013) Deciphering the cryptic genome: genome‐wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog 9: e1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters, P.J. , Faino, L. , van den Bosch, T. , Evenhuis, B. , Visser, R. , Seidl, M.F. , et al. (2018) Gapless genome assembly of the potato and tomato early blight pathogen Alternaria solani . Mol Plant Microbe Interact 31: 692–694. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Conner, R.L. , Wang, X. , Xu, R. , and Li, H. (2016) Variation in growth, colonization of maize, and metabolic parameters of GFP‐ and DsRed‐Labeled Fusarium verticillioides strains. Phytopathology 106: 890–899. [DOI] [PubMed] [Google Scholar]
- Wu, K.‐L. , Chen, W.‐Z. , Yang, S. , Wen, Y. , Zheng, Y.R. , Anjago, W.M. , et al. (2019) Isolation and identification of Fusarium oxysporum f. sp. cubense in Fujian Province, China. J Integr Agric 18: 1905–1913. [Google Scholar]
- Xing, H.Q. , Ma, J.C. , Xu, B.L. , Zhang, S.W. , Wang, L. , Cao, L. , et al. (2018) Mycobiota of maize seeds revealed by rDNA‐ITS sequence analysis of samples with varying storage times. Microbiologyopen 7: e00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Li, S. , and Liu, J. (2008) The development of Listeria monocytogenes Assay by PCR. Biotechnol Bull 1: 95–99+112. [Google Scholar]
- Yang, F. , Yang, J. , Zhang, X.B. , Chen, L.H. , Jiang, Y. , Yan, Y.L. , et al. (2005) Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res 33: 6445–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.C. , Haudenshield, J.S. , and Hartman, G.L. (2015) Multiplex Real‐time PCR detection and differentiation of Colletotrichum species infecting soybean. Plant Dis 99: 1559–1568. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Guo, X. , Wen, X. , and Xie, X. (2016) Rapid detection of three types of bacterial pathogens in cosmetics by multiplex PCR. Industrial Microbiol 46: 53–57. [Google Scholar]
- Yin, Y. , Liu, X. , and Ma, Z. (2009a) Simultaneous detection of Fusarium asiaticum and Fusarium graminearum in wheat seeds using a real‐time PCR method. Lett Appl Microbiol 48: 680–686. [DOI] [PubMed] [Google Scholar]
- Yin, Y. , Lou, T. , Yan, L. , Michailides, T.J. , and Ma, Z. (2009b) Molecular characterization of toxigenic and atoxigenic Aspergillus flavus isolates, collected from peanut fields in China. J Appl Microbiol 107: 1857–1865. [DOI] [PubMed] [Google Scholar]
- Yin, H.B. , Chen, C.H. , Kollanoor‐Johny, A. , Darre, M.J. , and Venkitanarayanan, K. (2015) Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans‐cinnamaldehyde. Poult Sci 94: 2183–2190. [DOI] [PubMed] [Google Scholar]
- Yoshida, T. , and Ichishima, E. (1995) Molecular‐cloning and nucleotide‐sequence of the genomic dna for 1,2‐alpha‐d‐mannosidase gene, msdc from penicillium‐citrinum. Biochim Biophys Acta Gene Struct Express 1263: 159–162. [DOI] [PubMed] [Google Scholar]
- You, M.P. , Lanoiselet, V. , Wang, C.P. , and Barbetti, M.J. (2014) First report of Alternaria leaf spot caused by Alternaria tenuissima on Blueberry (Vaccinium corymbosum) in Western Australia. Plant Dis 98: 423. [DOI] [PubMed] [Google Scholar]
- Yousaf, S. , Bulgari, D. , Bergna, A. , Pancher, M. , Quaglino, F. , Casati, P. , et al. (2014) Pyrosequencing detects human and animal pathogenic taxa in the grapevine endosphere. Front Microbiol 5: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, K. , Ahmed, Y. , Ligorio, A. , D'Onghia, A.M. , Nigro, F. , and Ippolito, A. (2010) First report of Penicillium ulaiense as a postharvest pathogen of orange fruit in Egypt. New Disease Rep 59: 1174–1174. [Google Scholar]
- Yu, J. , Perng‐Kuang, C. , Ehrlich, K.C. , Cary, J.W. , Deepak, B. , Cleveland, T.E. , et al. (2004) Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.J. , Jurick, W.M., II , Cao, H.S. , Yin, Y. , Gaskins, V.L. , Losada, L. , et al. (2014) Draft genome sequence of Penicillium expansum strain R19, which causes postharvest decay of apple fruit. Genome Announc 2: e00635‐00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. , Hu, X. , Deng, W. , Li, Y. , Han, G. , and Ye, C. (2016) Soil fungal community comparison of different mulberry genotypes and the relationship with mulberry fruit sclerotiniosis. Sci Rep 6: 28365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinfar, H. , Mirhendi, H. , Fata, A. , Khodadadi, H. , and Kordbacheh, P. (2015) Detection of Aspergillus flavus and A. fumigatus in bronchoalveolar lavage specimens of hematopoietic stem cell transplants and hematological malignancies patients by real‐time polymerase chain reaction, nested PCR and mycological assays. Jundishapur J Microbiol 8: e13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Zhu, Z. , Ma, Z. , and Li, H. (2009) A molecular mechanism of azoxystrobin resistance in Penicillium digitatum UV mutants and a PCR‐based assay for detection of azoxystrobin‐resistant strains in packing‐ or store‐house isolates. Int J Food Microbiol 131: 157–161. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Chen, Z. , Hou, Y. , Duan, Y. , Wang, J. , Zhou, M. , et al. (2015) PIRA‐PCR FOR Detection of Fusarium fujikuroi genotypes with carbendazim‐resistance alleles. Plant Dis 99: 1241–1246. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Chen, X. , Zhong, Q. , Huang, Z. , Meng, Z. , Luo, J. , et al. (2017a) Microbial communities on the wine grape surfaces of different cultivars. Biotechnol Bull 33: 128–137. [Google Scholar]
- Zhang, S.W. , Chen, X. , Zhong, Q.D. , Huang, Z.B. , and Bai, Z.H. (2017b) Relations among epiphytic microbial communities from soil, leaves and grapes of the grapevine. Front Life Sci 10: 73–83. [Google Scholar]
- Zhang, Y. , Li, X. , Shen, F. , Xu, H. , Li, Y. , and Liu, D. (2018) Characterization of Botrytis cinerea isolates from grape vineyards in China. Plant Dis 102: 40–48. [DOI] [PubMed] [Google Scholar]
- Zhou, Y.J. , Zhang, J. , Wang, X.D. , Yang, L. , Jiang, D.H. , Li, G.Q. , et al. (2014) Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 106: 43–56. [DOI] [PubMed] [Google Scholar]
- Zur, G. , Shimoni, E. , Hallerman, E. , and Kashi, Y. (2002) Detection of Alternaria fungal contamination in cereal grains by a polymerase chain reaction‐based assay. J Food Prot 65: 1433–1440. [DOI] [PubMed] [Google Scholar]


