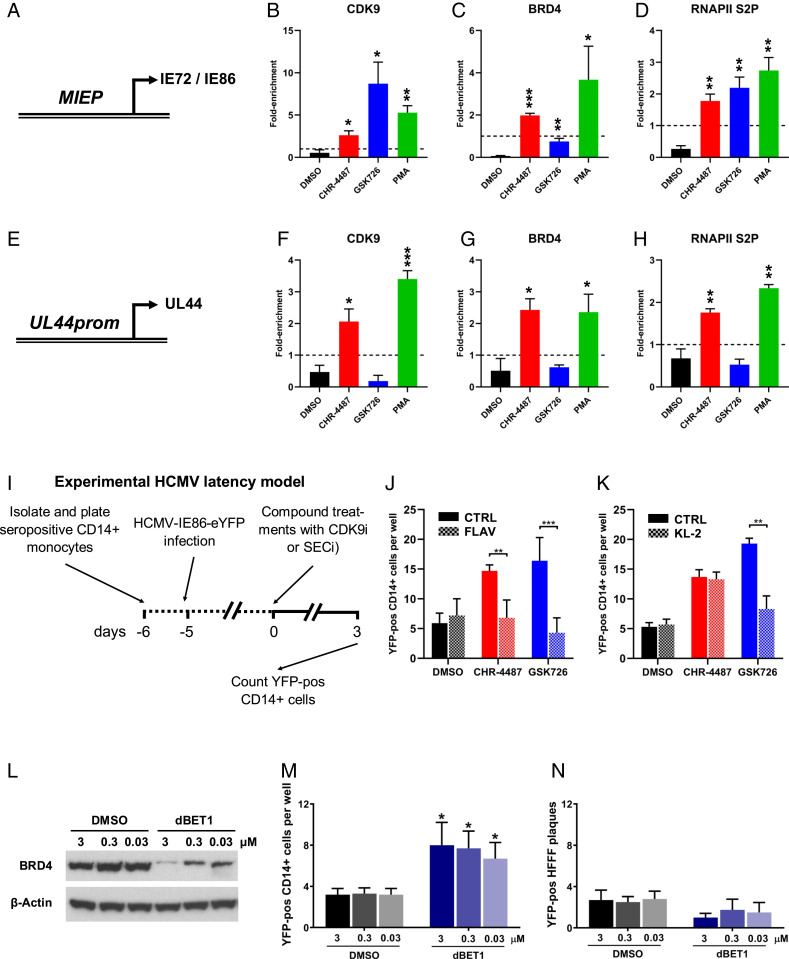
Fig. 4.
BRD4 inhibition modulates SEC:P-TEFb association with HCMV promoters. (A–H) Monocytic THP-1 cells infected with HCMV TB40e IE86-eYFP for 3 d were treated with DMSO (black bars), HDACi (CHR-4487 30 nM, red bars), I-BET (GSK726 30 nM, blue bars) or PMA (100 nM, green bars). After 48 h, ChIP qPCR analysis was employed to determine the enrichment of (A–D) MIEP and (E–H) UL44 promoter DNA using antibodies specific for (B and F) CDK9, (C and G) BRD4, and (D and H) RNAPII S2P relative to isotype controls (dashed line, set to 1) (mean + SEM, n = 3). (I) Treatment regime of cells in J–K. CD14+ monocytes isolated from apheresis cones infected with HCMV TB40e IE86-eYFP for 5 d were treated for 72 h with DMSO (black bars), HDACi (CHR-4487, red bars), or I-BET (GSK726, blue bars) (all 30 nM) with concurrent treatment of half of wells with either (J) CDK9 inhibitor (CDK9i), Flavopiridol (FLAV 40 nM), or (K) SECi KL-2 (10 μM) before the number of YFP-positive cells per well were then counted. (L–N) CD14+ monocytes isolated from apheresis cones infected with HCMV TB40e IE86-eYFP for 5 d were treated with DMSO (black bars) or BRD4-degrader dBET1 (blue bars) (3 to 0.03 μM) with immunoblot data confirming BRD4 degradation at 24 h (L) (images representative of n = 3). After 72 h, the number of YFP-positive cells per well then counted (M) before monocytes were overlaid with HFFFs and resulting plaques counted after 7 d (N) (mean + SEM, n = 5; *P < 0.05, **P < 0.01, ***P < 0.001).