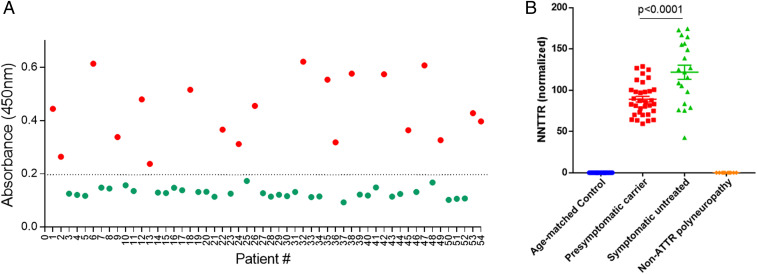
Fig. 3.
NNTTR-Dx ELISA accurately and specifically identifies TTR FAP patients and presymptomatic V30M carriers using plasma samples. (A) The assay accurately distinguished 20 V30M TTR FAP patients (red circles) from 34 age-matched controls (green circles) in a set of blinded plasma samples. (B) NNTTR detection in plasma samples from groups of age-matched controls (n = 34), presymptomatic V30M TTR mutation carriers (n = 33), symptomatic pretreatment V30M TTR FAP patients (n = 20), and non-TTR polyneuropathy patients (n = 10, including 3 with diabetic neuropathy, 4 with chronic inflammatory demyelinating polyneuropathy, and 4 with idiopathic neuropathy). NNTTR values are presented as relative values normalized to a pooled pretreatment FAP sample (Materials and Methods). Error bars represent SEM. P values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. All plasma samples used in the experiment are from the Amyloidosis Reference Center (Porto, Portugal), except for the non-TTR polyneuropathy samples, which are from the United States.