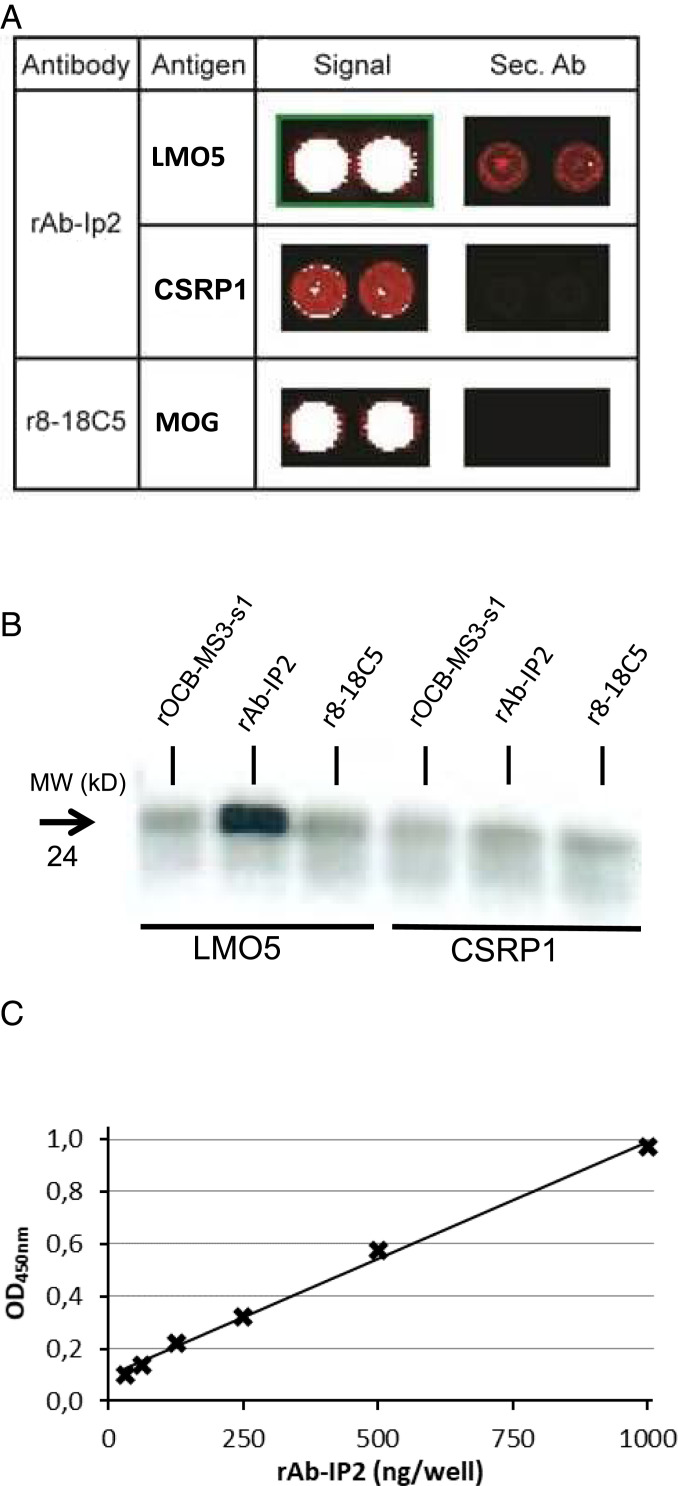
Fig. 3.
Identification of LMO5 as antigen of Ab-IP2. (A) Hybridization of rAb-IP2 to a protein array. Sections of the developed array are shown. The first column lists the antibodies used, the second column the detected target antigens, the third column the signals of the antigens, and the fourth column the signals of the secondary antibody alone. The color code ranges from black (no reactivity) to red (medium reactivity) and white (strong reactivity). All samples were spotted in duplicate. The upper row shows the detection of LMO5 (synonym CSRP2) by rAb-IP2. The middle row shows the detection of the homologous CSRP1 by rAb-IP2. Both proteins are recognized specifically as the secondary antibody alone yields no or a much weaker signal on the array. The lowest row shows recognition of the positive control antigen major oligodendrocyte glycoprotein (MOG) by the MOG-specific antibody r8-18C5. LMO5 and MOG were detected with high affinity, whereas CSRP1 was detected with low affinity. Reprinted with permission from ref. 32. (B) Validation of LMO5 recognition by rAb-IP2 by immunoprecipitation. Recombinant proteins LMO5 (lanes 1–3) and CSRP1 (lanes 4–6) were produced in HEK293 cells and precipitated with antibodies rOCB-MS3-s1 (lanes 1 and 4), rAb-IP2 (lanes 2 and 5), and r8-18C5 (lanes 3 and 6). Only LMO5 could be precipitated by rAb-IP2. The blot is representative for three independent experiments. (C) Validation of LMO5 recognition by rAb-IP2 by ELISA. LMO5 was coated to plates and detected by rAb-IP2 between 0 and 1,000 µg/mL rAb-IP2 recognition of recombinant LMO5 produced in E. coli occurred in a dose-dependent manner. The shown ELISA is representative for two independent experiments. The parameter for the linear equation is y = 0,0009x + 0,0942, and the correlation coefficient is R2 = 0.9954 with P < 0.001.