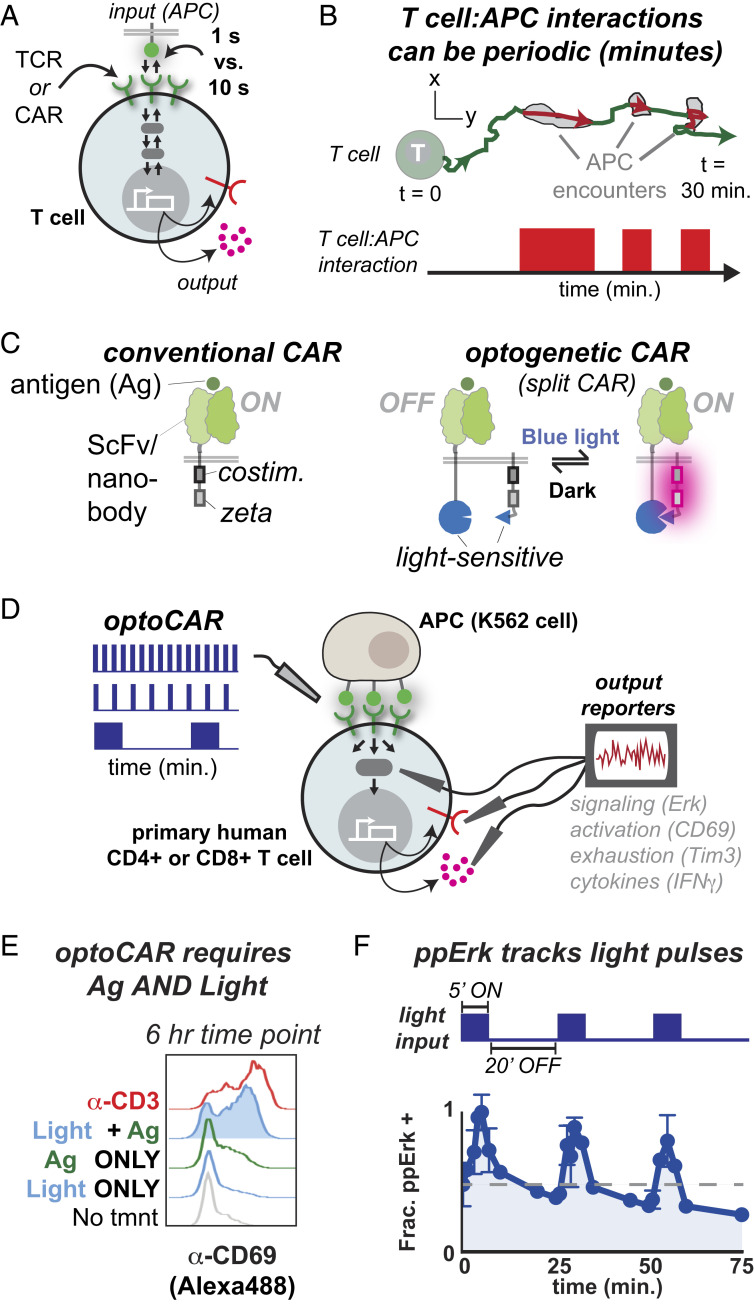
Fig. 1.
Systematically probing the transmission of periodic signals in T cells using optogenetics. (A) Dynamic receptor–ligand interactions regulate T cell gene expression by initiating reversible signaling and transcriptional cascades. Differences in receptor–ligand half-life as small as 1 s versus 10 s can result in dramatic differences in gene expression. (B) T cell–APC interactions can be highly periodic, introducing an element of T cell regulation on a longer, minutes timescale. (C, Left) A conventional CAR contains ITAM and costimulatory domains fused to a ligand-recognition domain, in this case an ScFv or nanobody. (C, Right) The optogenetic CAR is designed to split the ligand-recognition and signaling components of a CAR onto two separately expressed polypeptides, which are reversibly heterodimerized via light-sensitive domains. The system associates when blue light turns on and dissociates in the dark. (D) We used the optoCAR to stimulate primary human CD4+ T cells with temporally modulated waveforms and measured expression of cell surface receptors and cytokines. (E) The optoCAR induces CD69 expression at similar levels as the α-CD3 antibody OKT3 but only when light and antigen are present. Representative of over 10 independent experiments and seven donors. (F) Erk Thr202/Tyr204 phosphorylation turns on and off in response to several cycles of blue light illumination. The gray dashed line indicates initial ppErk levels. Representative of two independent experiments from one donor.