Figure 4.
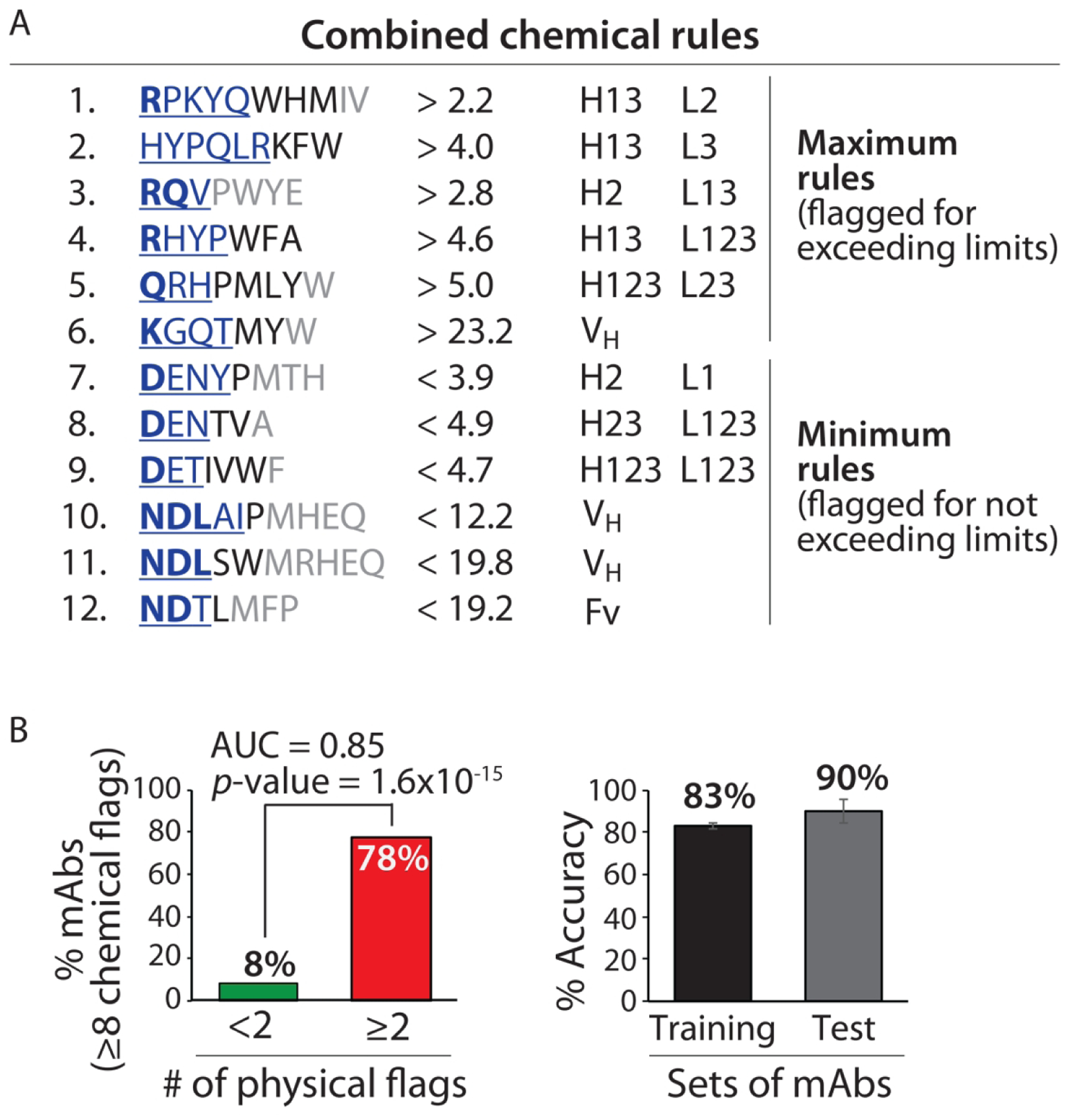
Combined chemical rules display high selectivity for identifying clinical-stage mAbs with low specificity. (A) Antibodies with predicted high specificity are required to be flagged by <8 of 12 rules. The contributions of the residues to each rule are reported as described in Figs. 2 and 3. (B) The combined rules selectively flag mAbs with low specificity (⩾2 physical flags) and display similar average adjusted accuracies for the training and test sets. The experimentally determined antibody specificities – as judged by five measurements of non-specific and self-interactions – are defined as described in Fig. 1. The p-values and adjusted accuracies were calculated as described in Fig. 2, and the area under the curve (AUC) is also reported.
