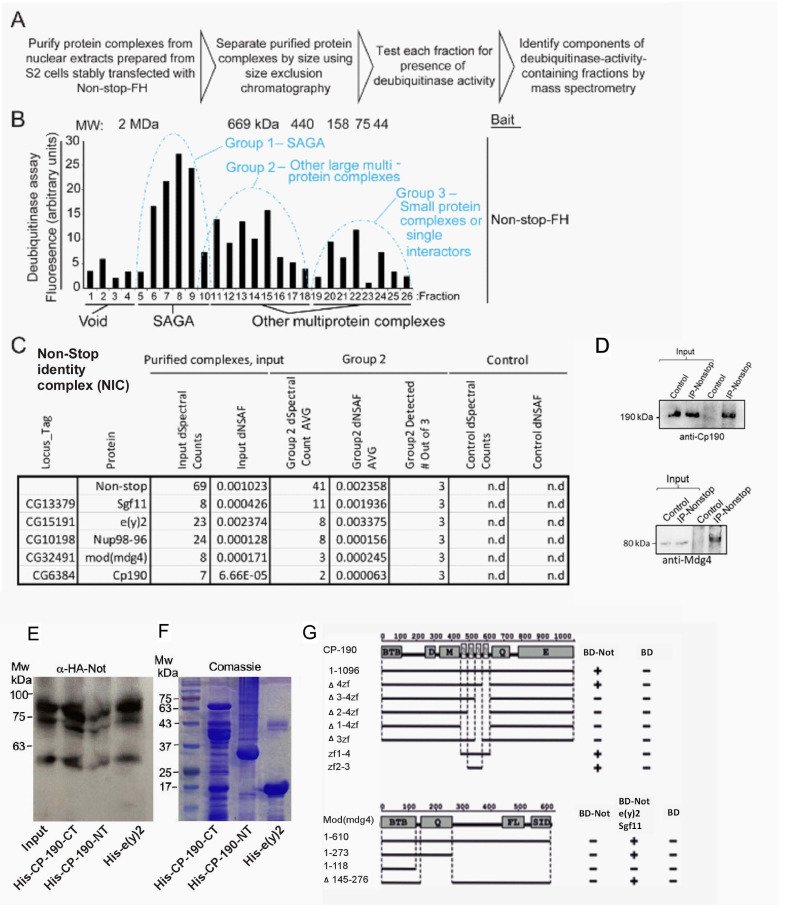
Figure 3. Identification of a Non-stop-identity complex (NIC).
(A–C) Purification scheme of nuclear Not-associated complexes from Drosophila S2 cells. (see text and methods; Reproduced from Figure 1B and E, Cloud et al., 2019), eLife, published under the Creative Commons Attribution 4.0 International Public License (CC BY 4.0; https://creativecommons.org/licenses/by/4.0/). (B) Identification of Not-associated isopeptidase activity proteins by immunoprecipitation followed by size fractionation and mass-spectrometry. CP190, Mod (mdg4), Nup96-98, and E(y)two were all present in Group 2. Not-FH; IP with full length Not FLAG-HA tagged (C) Summary of protein complexes isolated identified by mass-spectrometry (D, E) Not binds to the C-terminal portion of CP190 and to E(y)2. (D) Immunoprecipitation confirms Non-stop specifically interacts with NIC subunits Cp190 and Mdg4. Endogenous Non-stop was immunoprecipitated from whole cell extracts prepared from adult OregonR flies using an α-Non-stop antibody. Control immunoprecipitations were performed with α-guinea-pig IGG. The presence of NIC subunits was assayed by immunoblotting with antibodies specific for Cp190 or Mdg4 as indicated. (E) Western-blot of in vitro binding between HA-Not derived from S2 cell extract and the indicated bacterially expressed purified His-tagged proteins. 10% input is shown. (F) Coomassie blue staining of the indicated bacterially expressed His-tagged proteins used in the binding assay in (E). (G) Upper panel: Schematic diagram of Y2H interaction assay between CP190 and Non-stop. Different fragments of CP190 were fused to the activation domain (AD) of GAL4 and tested for interaction with Non-stop fused to the DNA-binding domain (BD) of GAL4. Protein domains of full-length CP190 are indicated as boxes, and lines represent the different deletion fragments. Zf denote zinc-fingers; BTB, BTB/POZ domain; D, aspartic acid -rich region; M, microtubule-interacting region; E, acid glutamate-rich region of CP190. The results are summarized in columns on the right (BD-Not and BD alone), with the ‘+” and “- “signs denotes presence and absence of interaction, respectively. Lower panel: Schematic diagram of Y2H and Y4H interaction assay between Mod(mdg4) and Non-stop. Different fragments of Mod(mdg4) were fused to the activation domain (AD) of GAL4 and tested for interaction with Non-stop fused to the DNA-binding domain (BD) of GAL4 and complex of BD-Non-stop with Eny2 and Sgf11. Protein domains of full-length Mod(mdg4) are indicated as boxes, and lines represent the different deletion fragments. BTB, BTB/POZ domain; Q, glutamine-rich region; FL, FLYWCH-type zinc finger domain; SID, Su(Hw) interaction domain. The results are summarized in columns on the right (BD-Not, BD-Not/Eny2/Sgf11 and BD alone), ‘+” and “- “signs denotes presence and absence of interaction, respectively.